25. C-13 and 2D NMR. Electrophilic Aromatic Substitution
Freshman Organic Chemistry II (CHEM 125B)
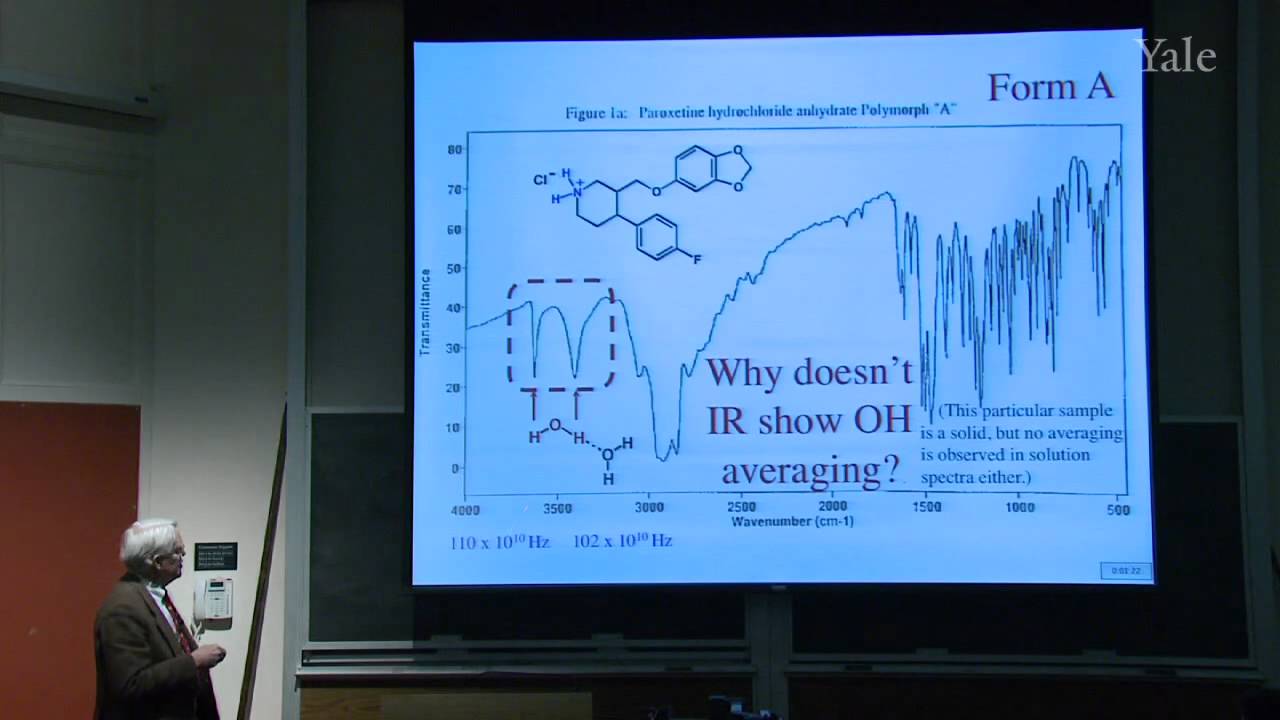
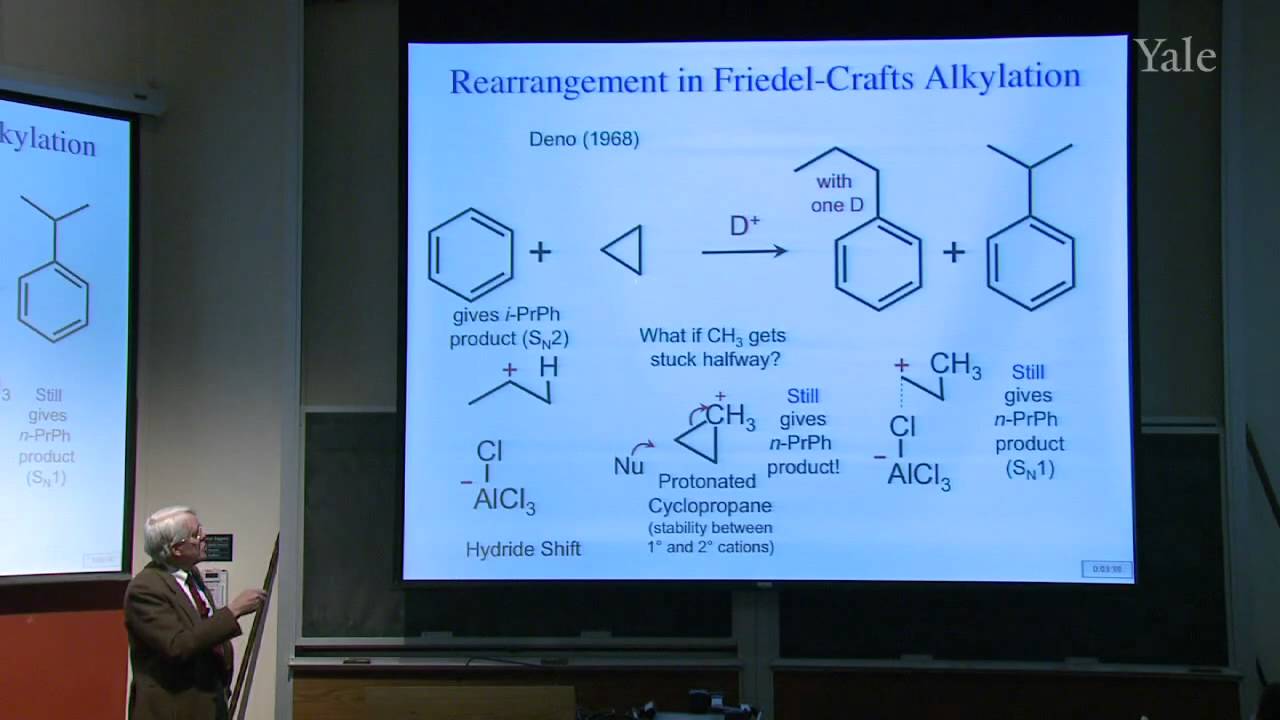
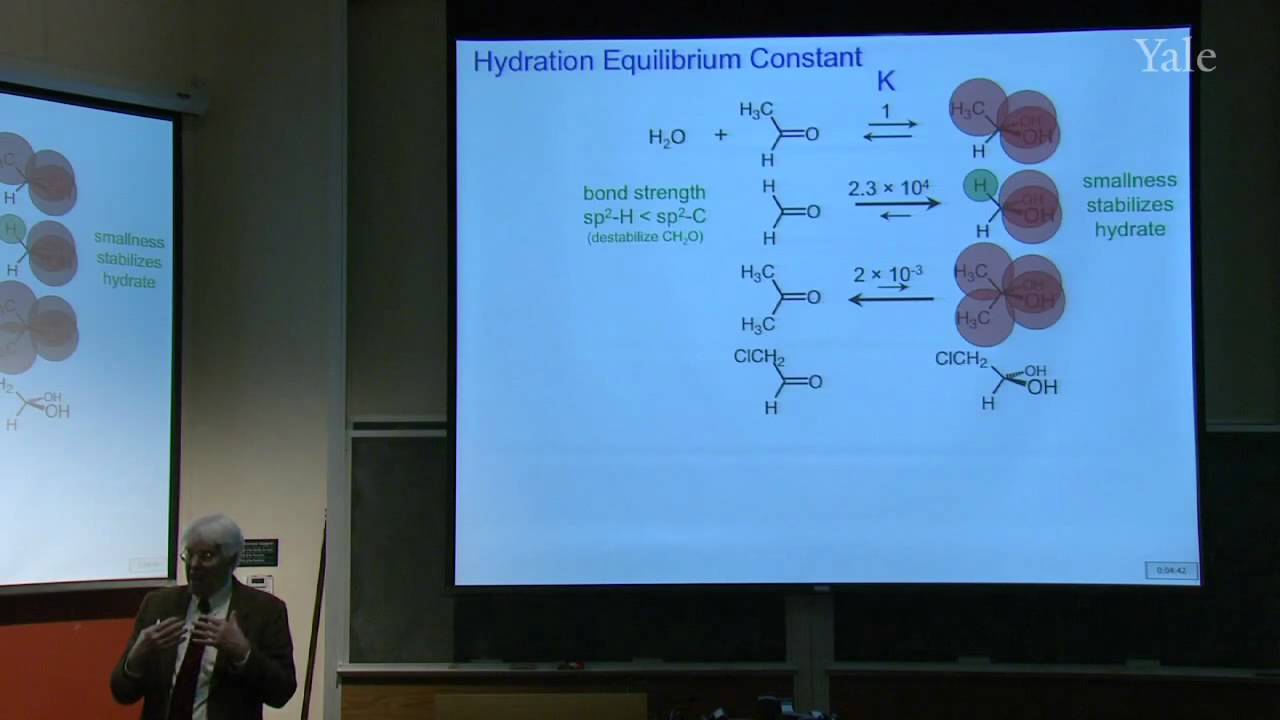
Proton decoupling simplifies C-13 NMR spectra. Dilute double labeling with C-13 confirmed the complex rearrangement scheme in steroid biosynthesis. Two-dimensional NMR yields correlations between NMR signals that underlie structural determination of proteins and identification of the mechanism of a rapid carbocation rearrangement. Substitution of an electrophile for a proton on an aromatic ring proceeds by a two-step association-dissociation mechanism involving a cyclohexadienyl cation intermediate. The relative rates of forming various products from substituted benzenes correlates with the substituents' influences on the stability of the various cyclohexadienyl cation intermediates. The spectrum of electrophile reactivities is very broad. Important contributions for activating electrophiles were made by Friedel and Crafts working in Paris.
00:00 - Chapter 1. Proton Decoupling
04:39 - Chapter 2. C-13 NMR: Double Labeling and Lanosterol Biosynthesis
19:51 - Chapter 3. 2-D NMR for Protein Structure and Rearrangement Rate
39:07 - Chapter 4. Electrophilic Aromatic Substitution: Substituent Effects
46:25 - Chapter 5. Electrophile Activation: Friedel and Crafts
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.



























![35. Acyl Insertions and [gr]α-Reactivity](https://i.ytimg.com/vi/Xb4CwzP-Hdk/maxresdefault.jpg)
![36. [gr]α-Reactivity and Condensation Reactions](https://i.ytimg.com/vi/DQfUaXLk_sw/maxresdefault.jpg)












![Chapter 5.6: Substitution & area between curves [lecture 27/29]](https://i.ytimg.com/vi/gPP3rP0CXnk/mqdefault.jpg)

