6. Brønsted Acidity and the Generality of Nucleophilic Substitution
Freshman Organic Chemistry II (CHEM 125B)
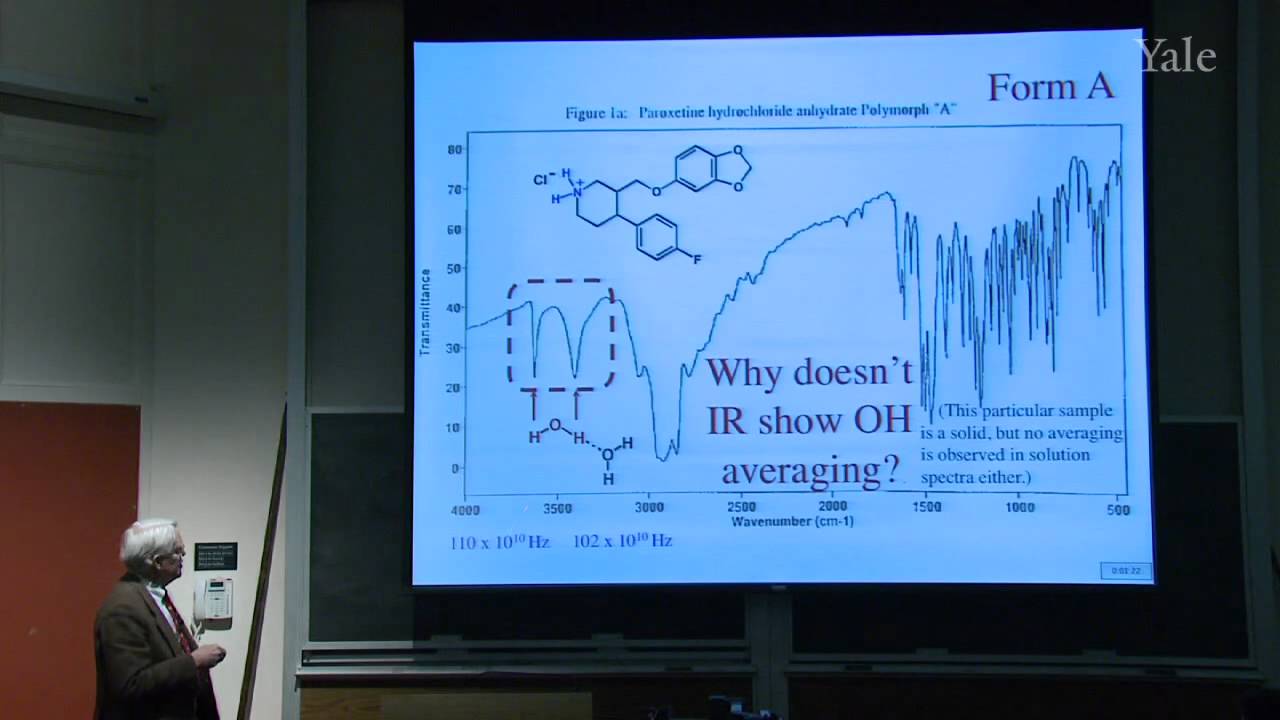
The coincidentally substantial extent of ionic dissociation of water provides an example of Brønsted acidity, or nucleophilic substitution at hydrogen. Relative pKa values are insensitive enough to solvent that they provide insight on the role of energy-match, overlap, and resonance in ionic dissociation. The titration of alanine in water illustrates the experimental determination of pKa values and the phenomenon of buffering. The limited pKa scale in water can be extended dramatically by titration in other solvents, providing one of the best ways to measure many "effects" in organic chemistry. A wide range of important organic reactions discovered in the 19th century and many biochemical reactions can be understood under the rubric of nucleophilic substitution.
00:00 - Chapter 1. Solvent Influence on Ionic Dissociation
04:45 - Chapter 2. Brønsted Acidity as Nucleophilic Substitution at Hydrogen
11:09 - Chapter 3. Understanding Relative pKa in Terms of Energy Match and Overlap
21:18 - Chapter 4. Measuring the pKa values of Alanine
29:09 - Chapter 5. Factors that Influence pKa over an Expanded Range
37:06 - Chapter 6. The Generality of Nucleophilic Substitution
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.










![Chapter 5.6: Substitution & area between curves [lecture 27/29]](https://i.ytimg.com/vi/gPP3rP0CXnk/mqdefault.jpg)