11. Carbocations and the Mechanism of Electrophilic Addition to Alkenes and Alkynes
Freshman Organic Chemistry II (CHEM 125B)
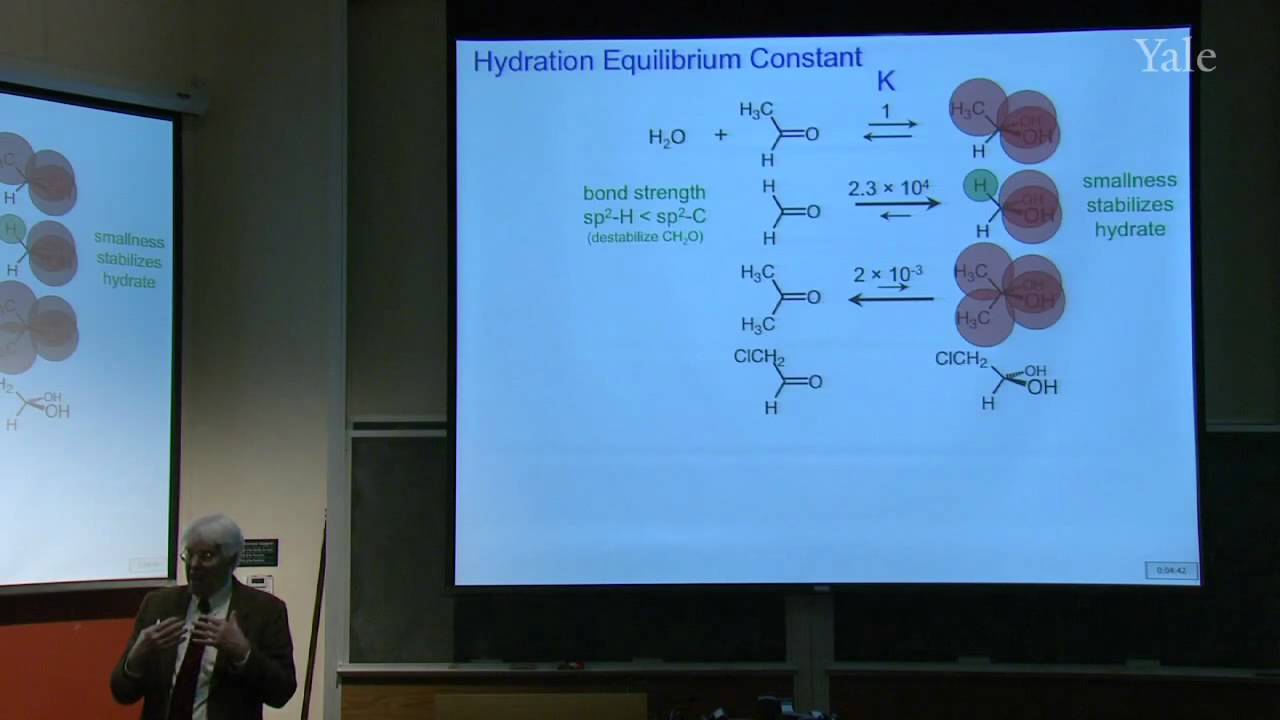
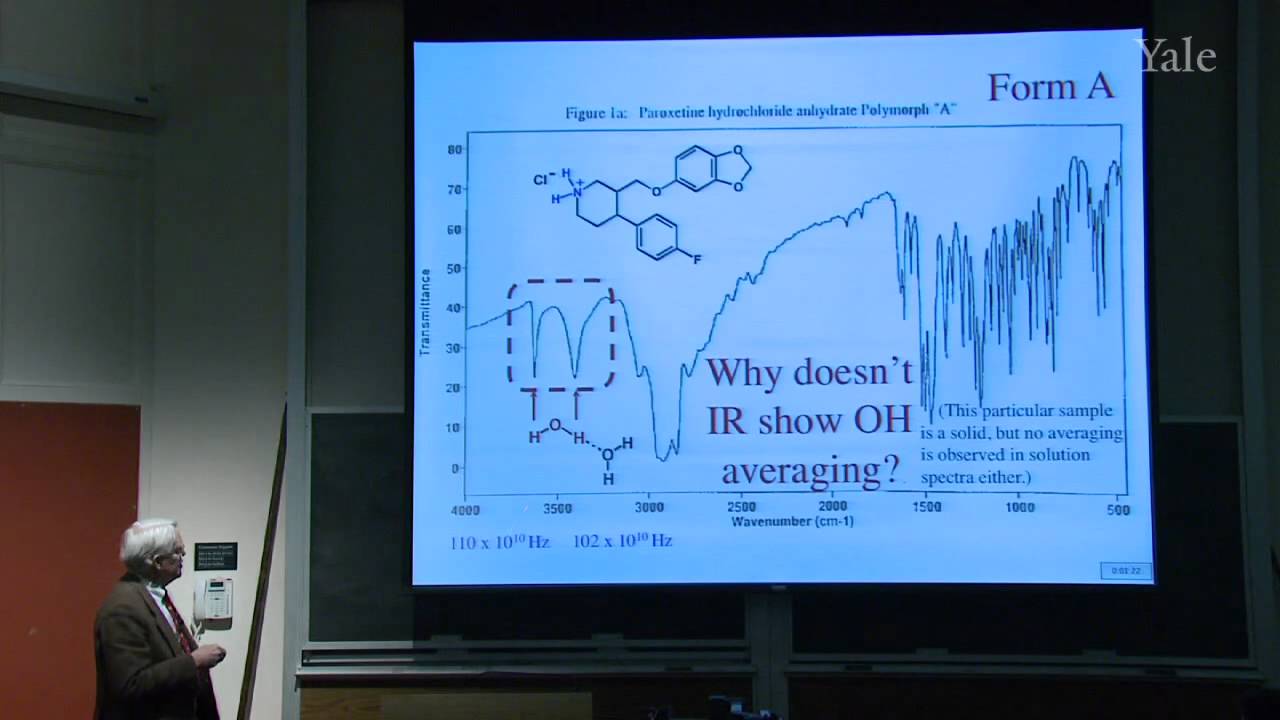
Substition stabilizes alkenes, and addition of acids is thermodynamically favorable in acidic media. Additon to alkenes can involve free-radical, metal-catalyzed, and stepwise electrophilic mechanisms, the last via a cation intermediate. Electrostatics can help position an attacking electrophile like H+, but bonding en route to Markovnikov addition requires orbital mixing to form the more stable cation. Relative cation stability can be understood in terms of hyperconjugation, hybridization, and solvation or polarizability. Stabilization of a carbocation via methide shift can compete with its trapping by solvent. The curious relative rates in stepwise addition of HCl or HBr to alkynes show that halogen substituents are both electron withdrawing and electron donating.
00:00 - Chapter 1. Alkene Thermochemistry
06:19 - Chapter 2. Alkene Addition Mechanisms
22:41 - Chapter 3. Understanding Carbocation Stabilities
39:01 - Chapter 4. Skeletal Rearrangement of Carbocations
43:16 - Chapter 5. Stepwise Addition to Alkynes -- Competing Influences of Halogen
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.