35. Acyl Insertions and [gr]α-Reactivity
Freshman Organic Chemistry II (CHEM 125B)
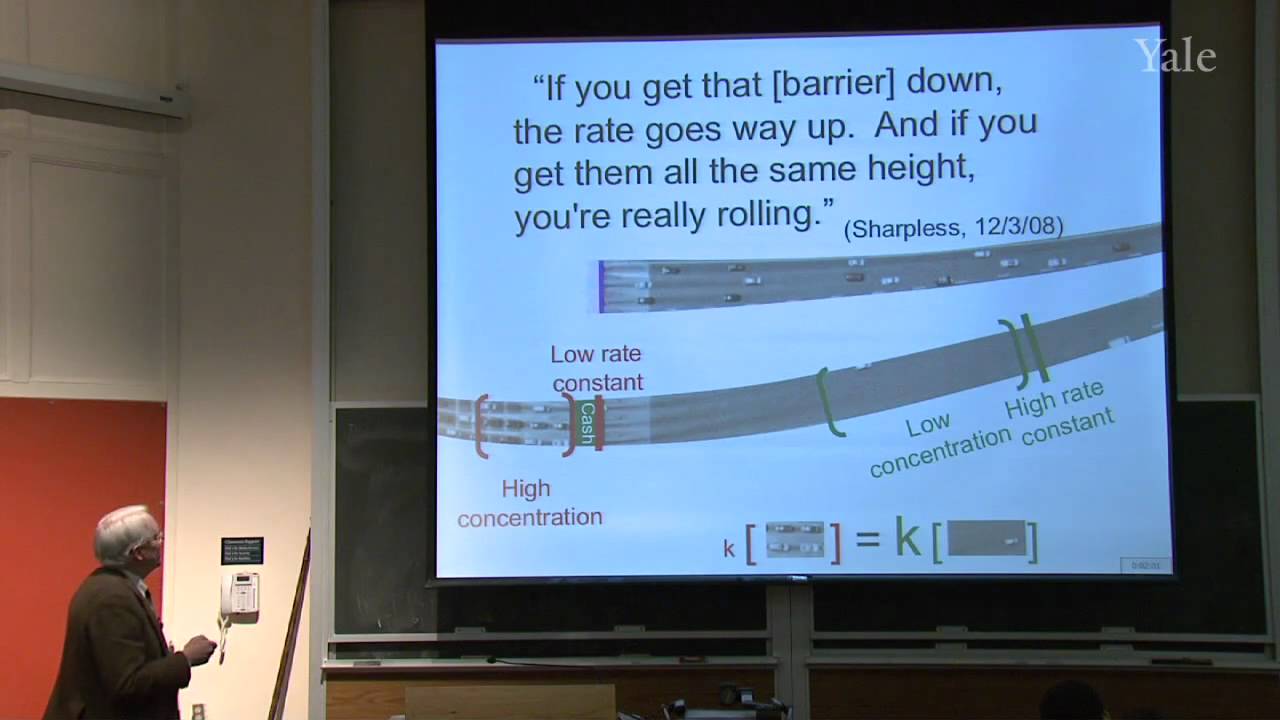
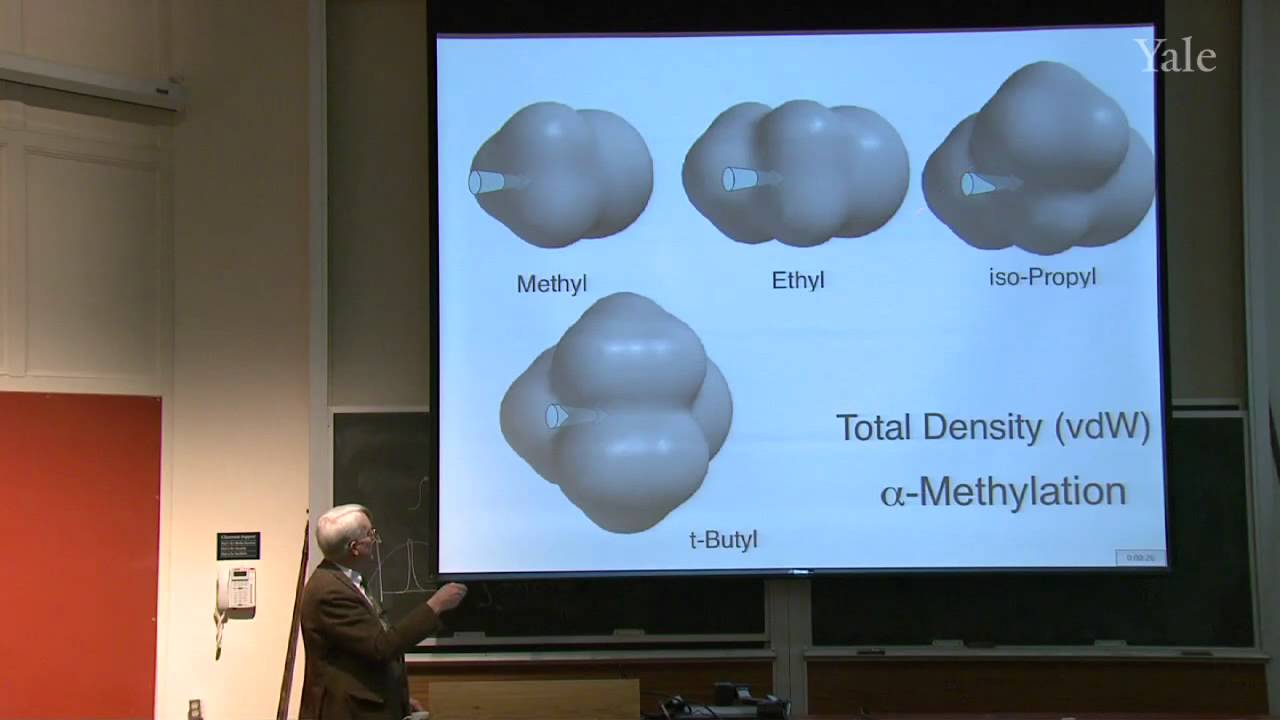
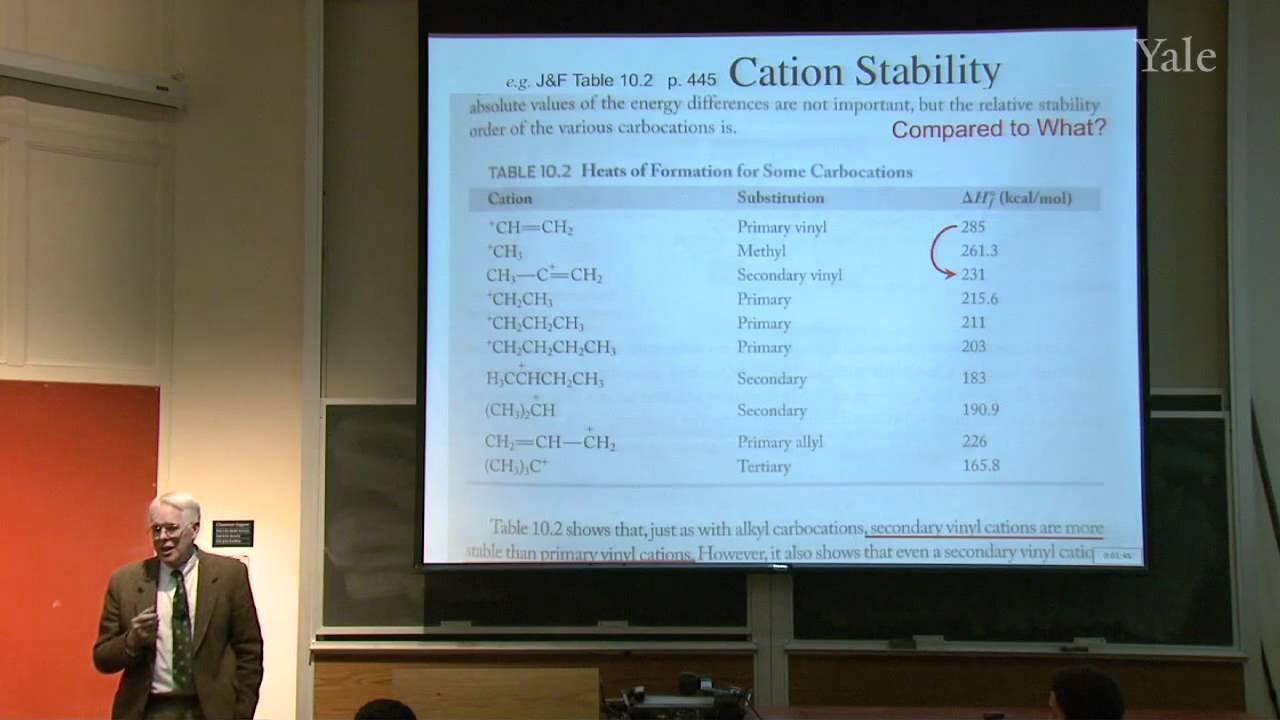
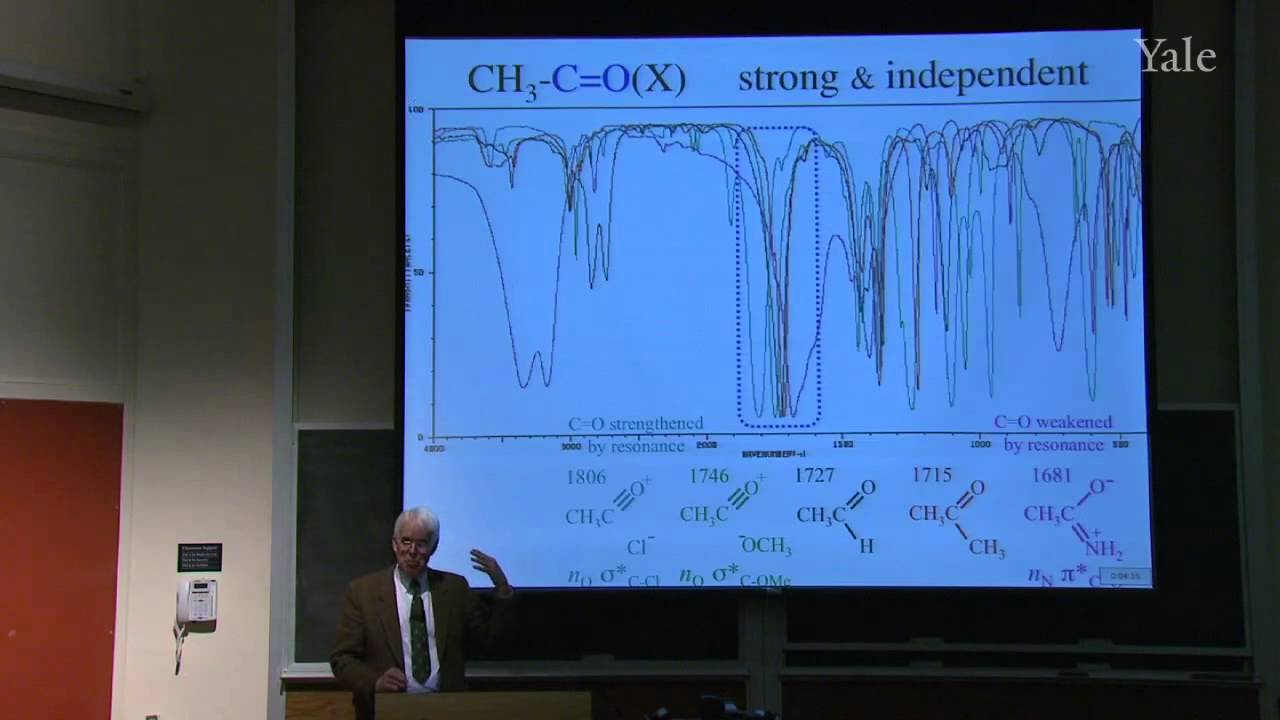
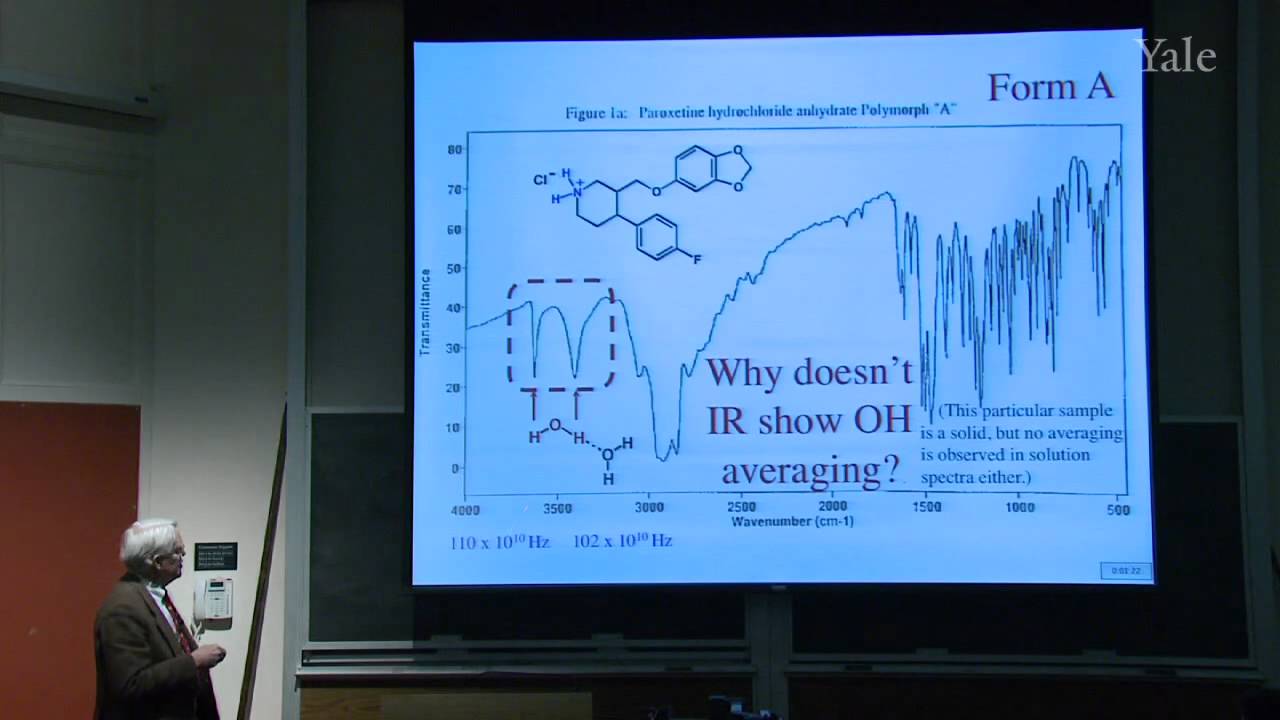
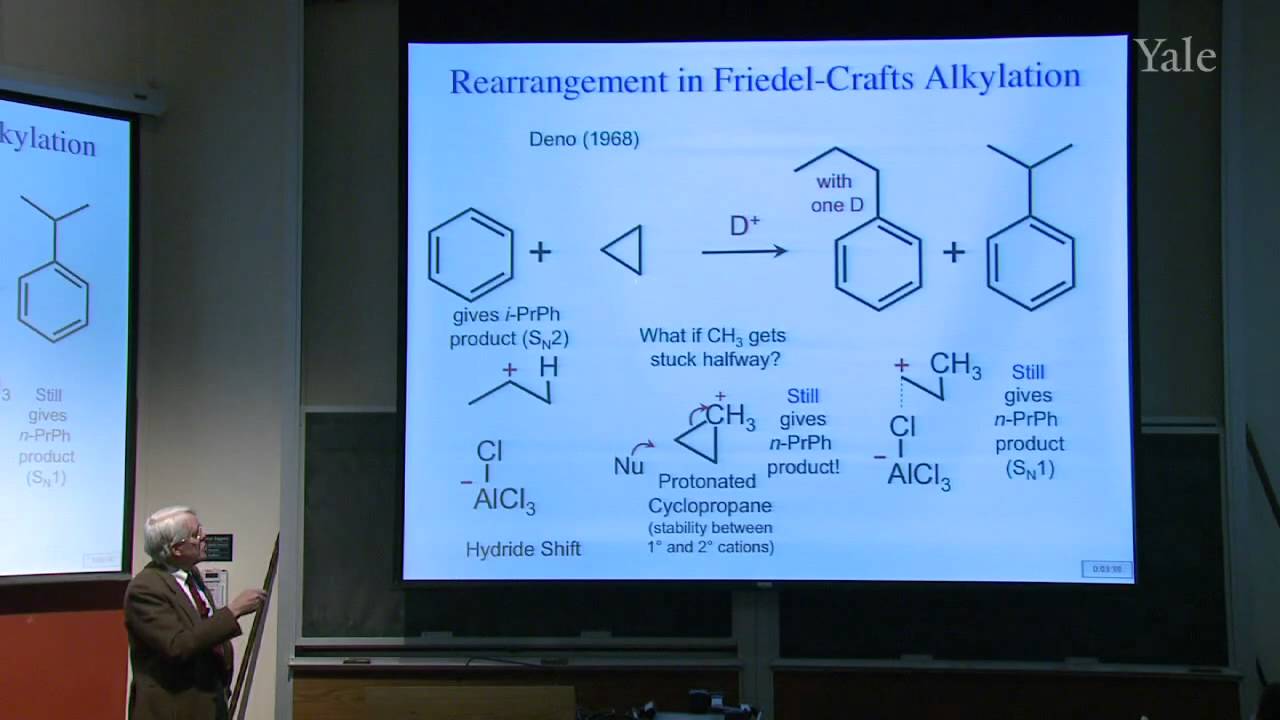
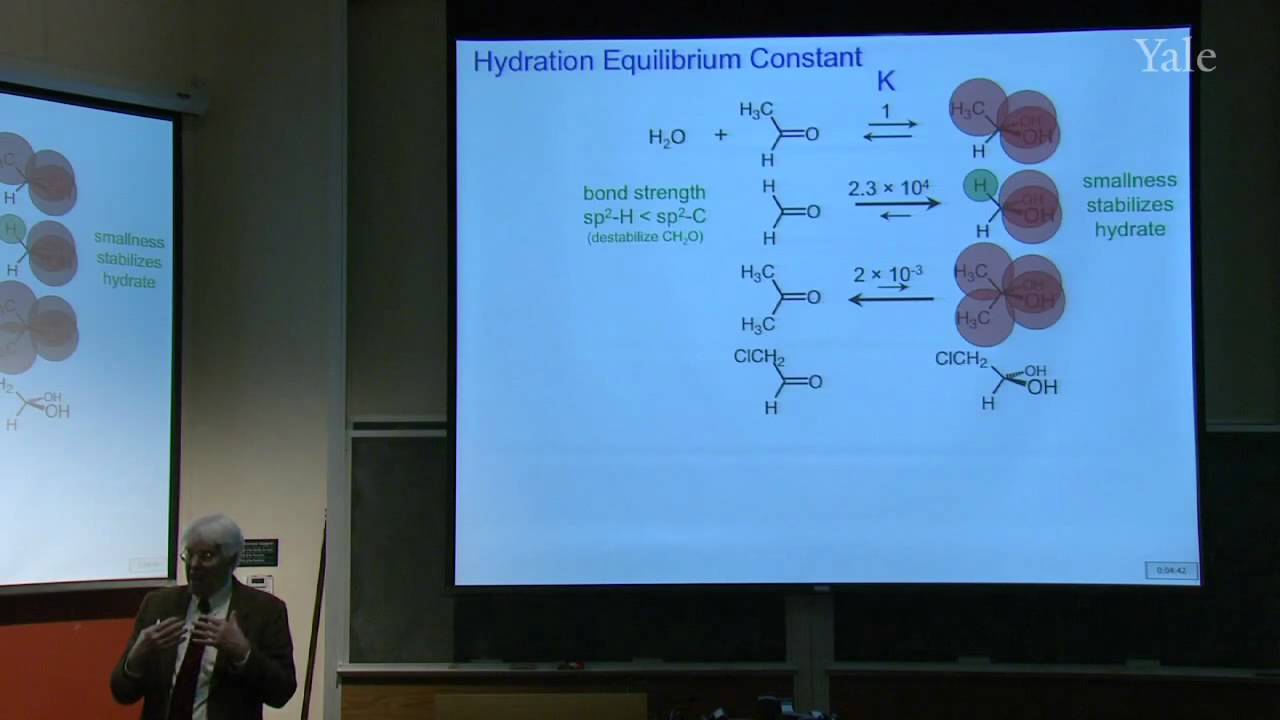
When a nucleophilic atom bearing a good leaving group attacks a carbonyl group, an adjacent R group can migrate to the new atom, inserting it into the R-acyl bond. This mechanism can insert O, NH, or CH2 groups into the acyl bond with informative stereospecificity in the case of the Beckmann rearrangement of oximes. Although the migrating groups are formally anionic, relative migratory aptitudes show that they give up electron density during rearrangement. Acid dissociation of protons [gr]α to a carbonyl group to form enolates, and the ease of forming enols, gives [gr]α-carbons nucleophilic reactivity under both basic and acidic conditions. This explains H/D exchange and racemization as well as halogenation and alkylation of [gr]α-carbons.
00:00 - Chapter 1. Acyl Insertion of O, NH, and CH2
25:29 - Chapter 2. A-Acidity
34:36 - Chapter 3. H/D Exchange and Racemization via Enol or Enolate
37:52 - Chapter 4. A-Halogenation
46:49 - Chapter 5. A-Alkylation
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.



























![35. Acyl Insertions and [gr]α-Reactivity](https://i.ytimg.com/vi/Xb4CwzP-Hdk/maxresdefault.jpg)
![36. [gr]α-Reactivity and Condensation Reactions](https://i.ytimg.com/vi/DQfUaXLk_sw/maxresdefault.jpg)








