16. Isoprenoids, Rubber, and Tuning Polymer Properties
Freshman Organic Chemistry II (CHEM 125B)
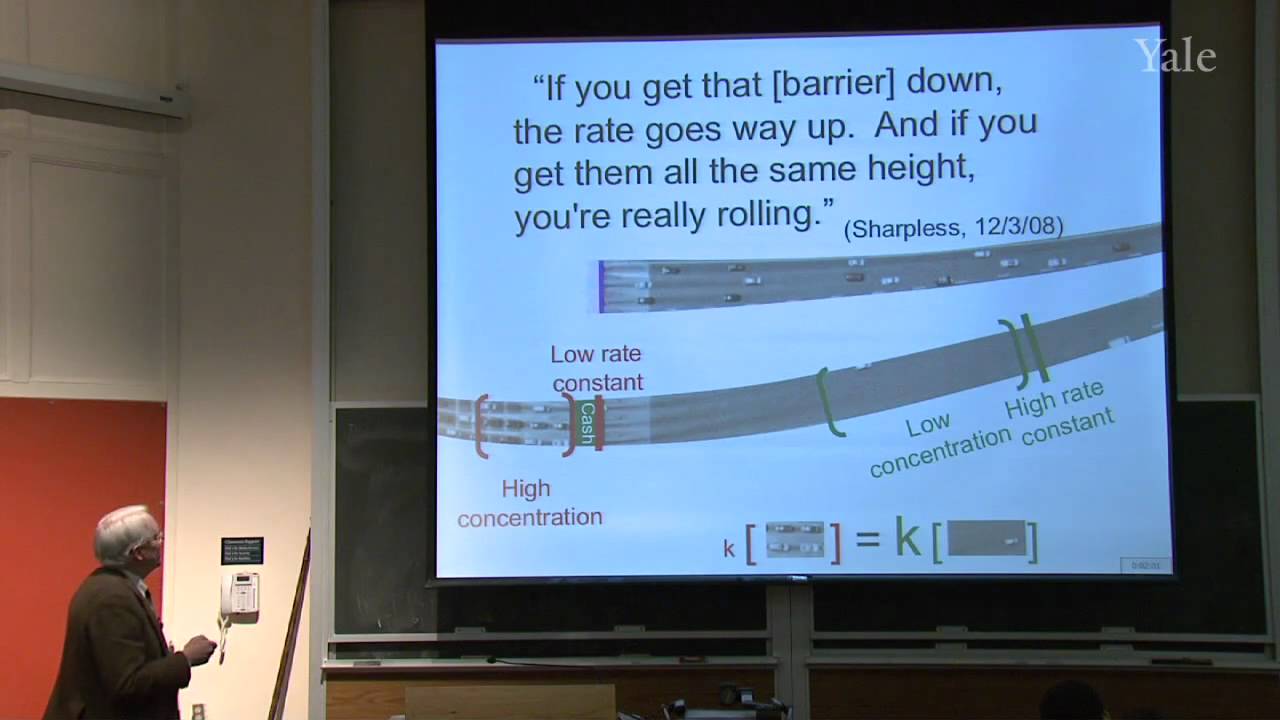
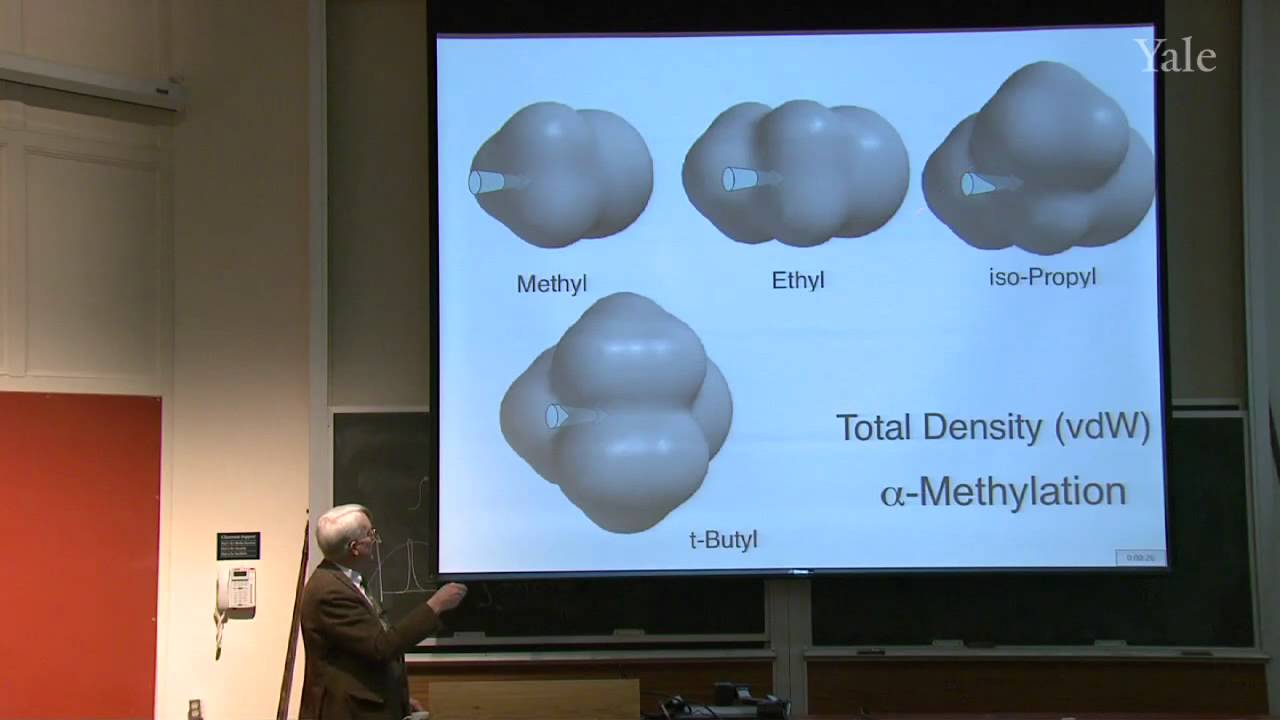
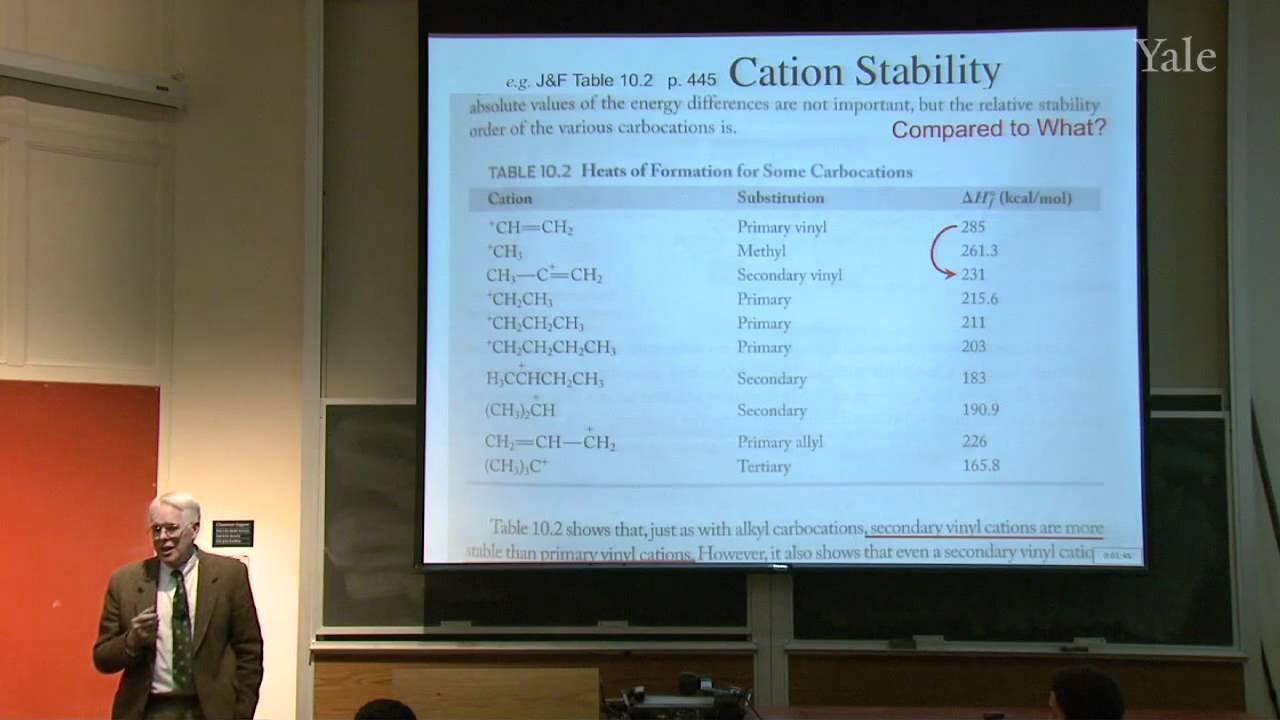
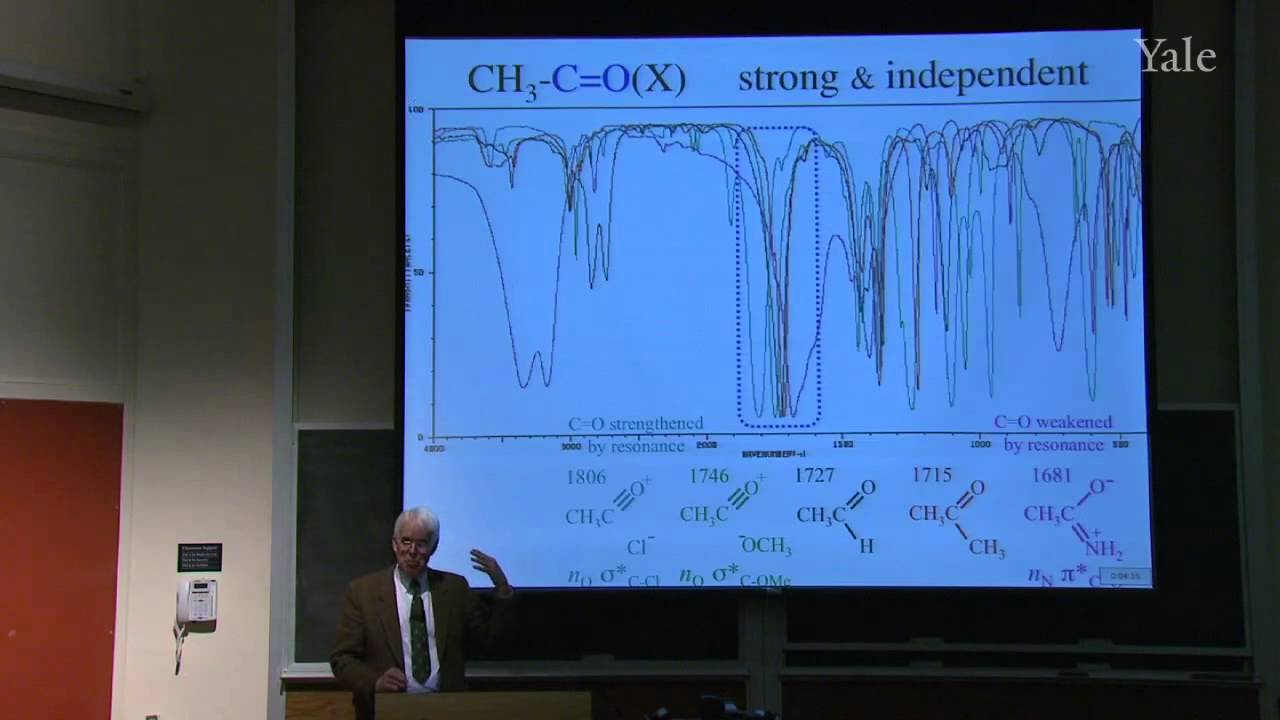
Isoprenoid or terpene natural products, that seem to be made from isoprene (2-methylbutadiene), are formed by oligomerization of electrophilic isopentenyl pyrophosphate (IPP). Latex, the polymer of IPP, became commercially important when Charles Goodyear, a New Haven native, discovered how to vulcanize rubber. Statistical mechanics explains such curious properties of rubber as contraction upon heating when tightly stretched. Specific chemical treatment confers useful properties on a wide variety of polymers, including hair, synthetic rubber, and plastics. The structure of copolymers demonstrates non-Hammond behavior and ionic character in the transition state for free-radical polymerization.
00:00 - Chapter 1. IPP as the Carbon Electrophile in Isoprenoid Biosynthesis
13:56 - Chapter 2. Latex, Rubber, and Vulcanization
20:14 - Chapter 3. Understanding Vulcanization - Polymer Properties and Statistical Mechanics
35:34 - Chapter 4. Other Polymers and Their Properties
38:22 - Chapter 5. Synthetic Polymers and Free-Radical Copolymerization
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.



























![35. Acyl Insertions and [gr]α-Reactivity](https://i.ytimg.com/vi/Xb4CwzP-Hdk/maxresdefault.jpg)
![36. [gr]α-Reactivity and Condensation Reactions](https://i.ytimg.com/vi/DQfUaXLk_sw/maxresdefault.jpg)




















