23. Diamagnetic Anisotropy and Spin-Spin Splitting
Freshman Organic Chemistry II (CHEM 125B)
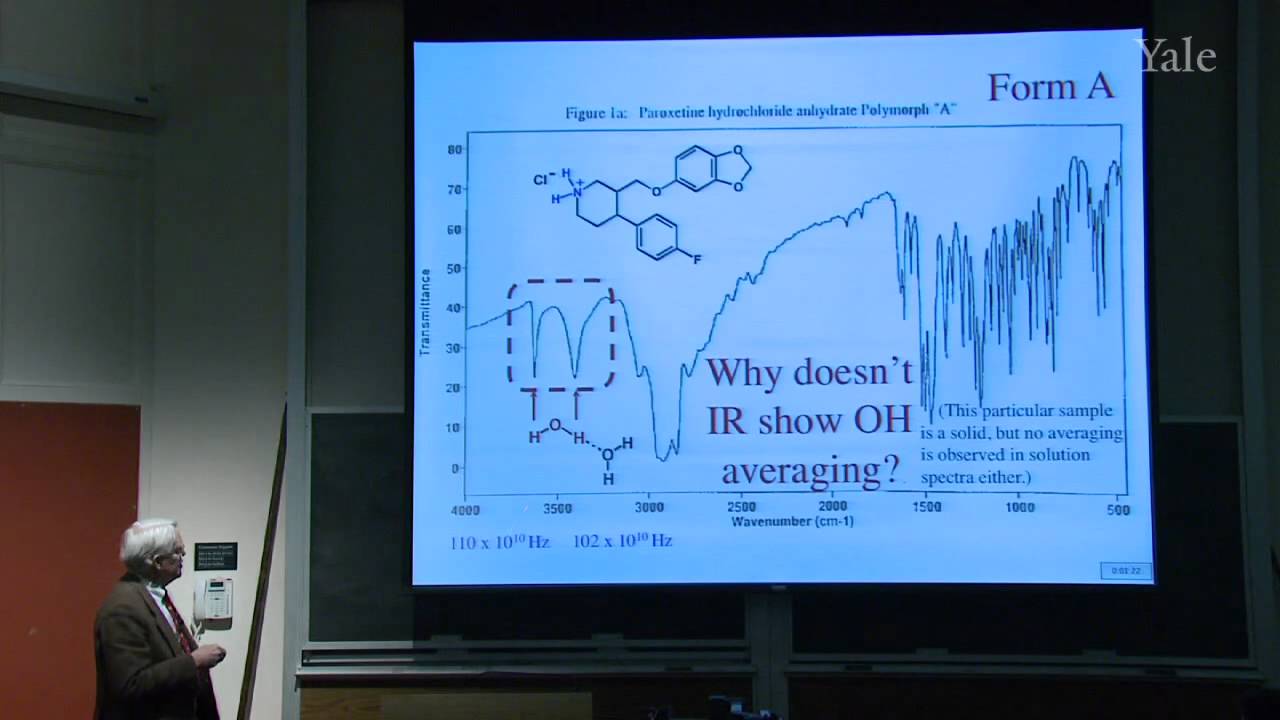
Through-space interaction between magnets of fixed strength and orientation averages to zero during random molecular tumbling, suggesting that the local field about a proton should be sensitive only to electrons that orbit about itself. The chemical shift can be sensitive to electrons orbiting elsewhere if the amount of orbiting varies with molecular orientation. This "diamagnetic anisotropy" is commonly used to rationalize the unusual chemical shifts of protons in acetylene and in aromatic and antiaromatic compounds. The other source of a proton's local field is nearby magnetic nuclei, which can be counted by the splitting multiplicity. Unlike chemical shift, which is measured in fractional units because it depends on the strength of the applied field, this spin-spin splitting (J), measured in Hz, is dependent only on molecular structure. J depends not on spatial proximity, but on orbital overlap, which, remarkably, is larger for anti- than for eclipsed conformations.
00:00 - Chapter 1. Diamagnetic Anisotropy and Aromaticity
20:50 - Chapter 2. Multiplicity in Spin-Spin Splitting
36:05 - Chapter 3. Tumbling, Orbitals, and the Magnitude of J
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.



























![35. Acyl Insertions and [gr]α-Reactivity](https://i.ytimg.com/vi/Xb4CwzP-Hdk/maxresdefault.jpg)
![36. [gr]α-Reactivity and Condensation Reactions](https://i.ytimg.com/vi/DQfUaXLk_sw/maxresdefault.jpg)





















