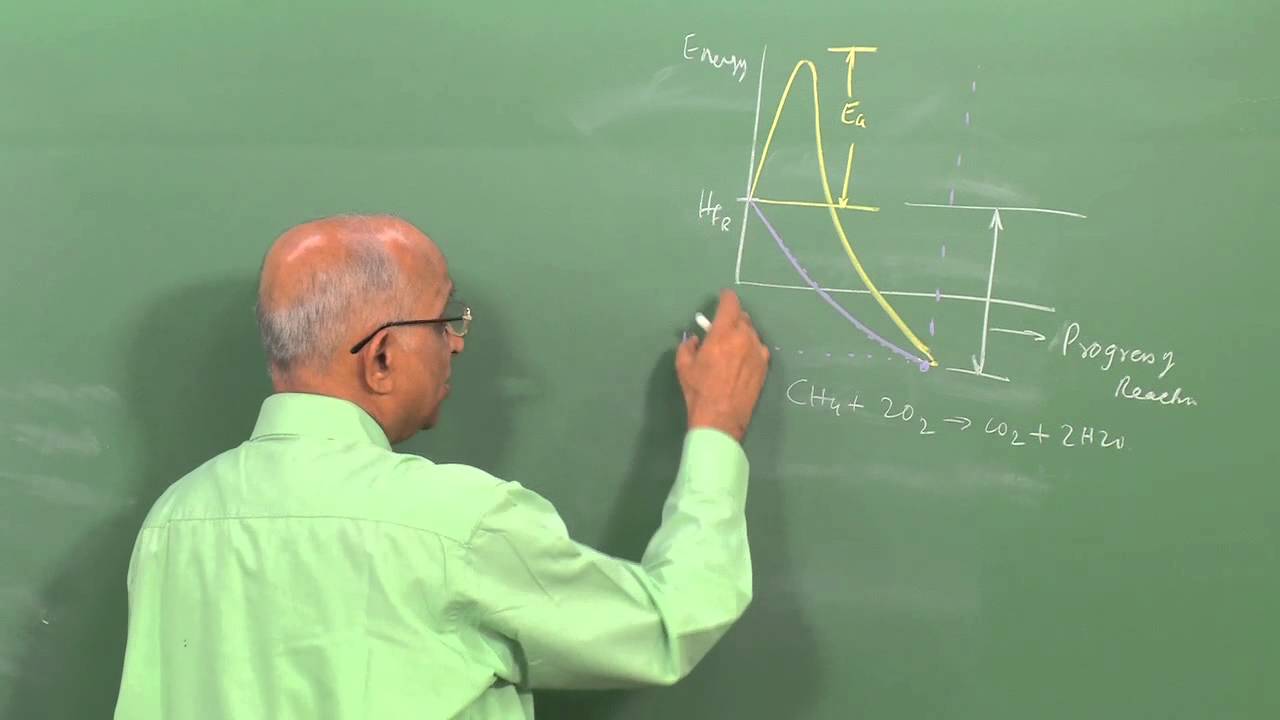7. Nucleophilic Substitution Tools - Stereochemistry, Rate Law, Substrate, Nucleophile
Freshman Organic Chemistry II (CHEM 125B)
SN2 substitution provides an example of establishing the mechanism of a chemical reaction by disproving all the alternatives. Five general pathways are envisioned (two-step involving either pentavalent or trivalent carbon intermediates, and one-step). They can be discriminated by applying a variety of experimental tools including stereochemistry (Walden inversion), rate law (second order and pseudo first order), and the variation of rate constant with changes in the substrate (steric hindrance and ring strain), and with changes in nucleophile or leaving group. Classic experiments by Kenyon and Phillips and by Bartlett and Knox established the nature of Walden inversion.
00:00 - Chapter 1. "Proving" a Mechanism by Imagining and Disproving All the Alternatives
06:03 - Chapter 2. Kenyon and Phillips Pinpoint Backside Attack in Nucleophilic Substitution
18:56 - Chapter 3. Using Kinetics to Study Mechanisms -- Rate Law
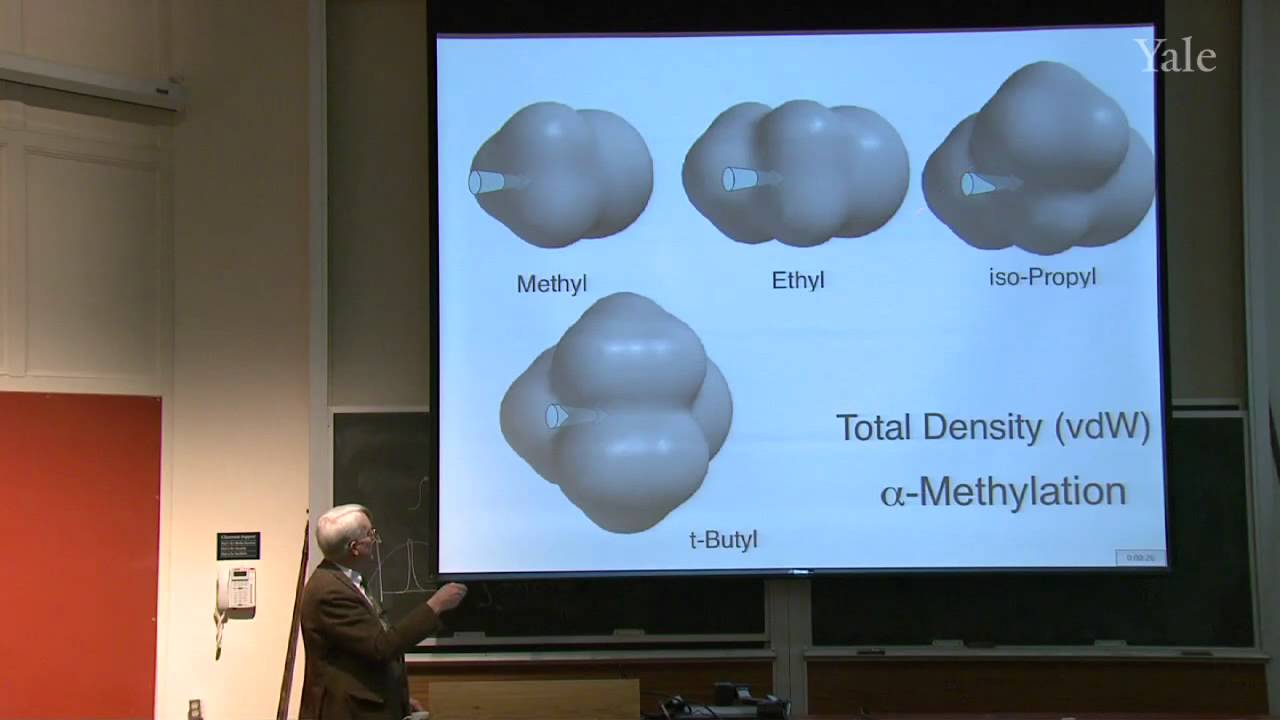
25:47 - Chapter 4. Rate Constant -- the Influence of Substrate Structure
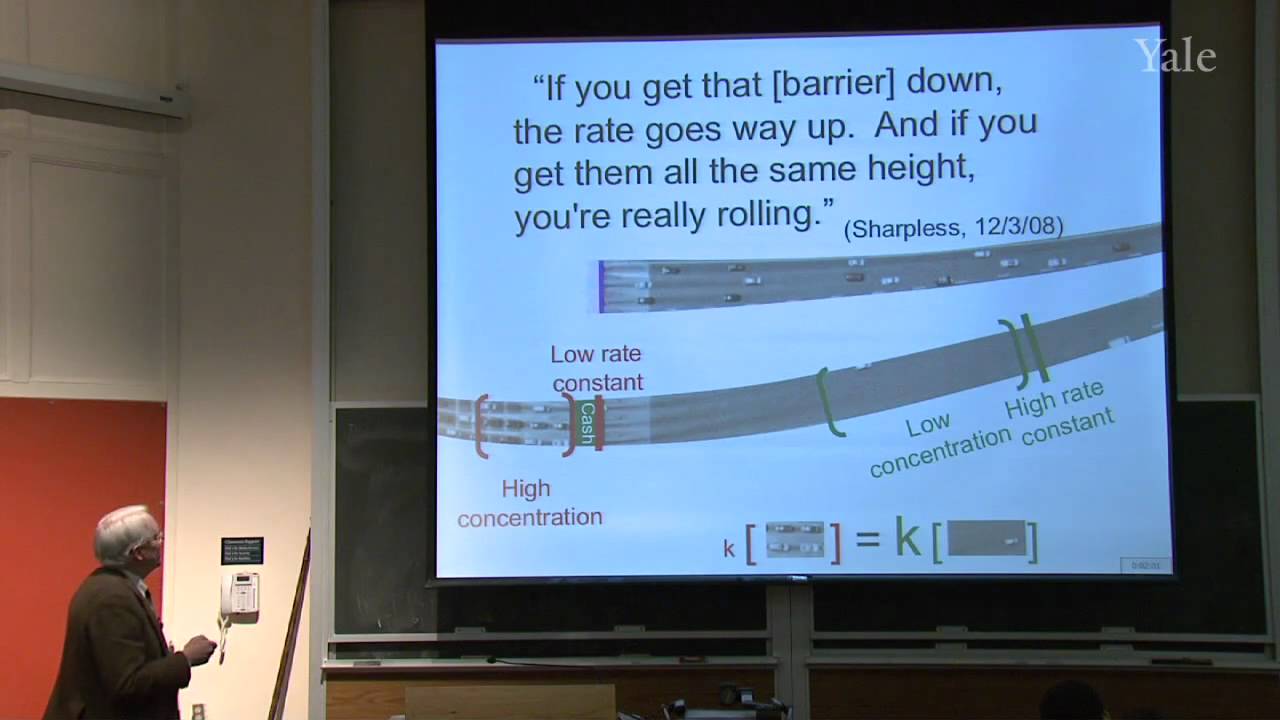
25:47 - Chapter 5. Rate Constant -- the Influence of Nucleophile and Leaving Group
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.



























![35. Acyl Insertions and [gr]α-Reactivity](https://i.ytimg.com/vi/Xb4CwzP-Hdk/maxresdefault.jpg)
![36. [gr]α-Reactivity and Condensation Reactions](https://i.ytimg.com/vi/DQfUaXLk_sw/maxresdefault.jpg)