19. Aromatic Transition States: Cycloaddition and Electrocyclic Reactions
Freshman Organic Chemistry II (CHEM 125B)
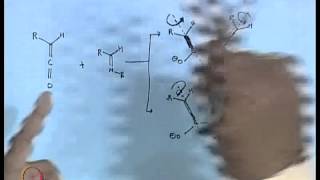
Cyclic conjugation that arises when p-orbitals touch one another can be as important for transition states as aromaticity is for stable molecules. It is the controlling factor in "pericyclic" reactions. Regiochemistry, stereochemistry, and kinetics show that two new sigma bonds are being formed simultaneously, if not symmetrically, in the 6-electron Diels-Alder cycloaddition. Although thermal dimerization of thymine residues in DNA is forbidden, photochemistry allows the 4-electron cycloaddition. "Electrocyclic" ring opening or closing chooses a conrotatory Möbius pathway, or a disrotatory Hückel pathway, according to the number of electron pairs involved and whether light is used in the process. Dewar benzene provides an example of a very unstable molecule that can be formed photochemically and then persists because of unfavorable orbital overlap in the transition state for ring opening.
00:00 - Chapter 1. Aromatic Ions
05:59 - Chapter 2. Pericyclic Reactions: Cycloaddition, the Diels-Alder Reaction, and Photochemistry
28:15 - Chapter 3. Electrocyclic Stereochemistry
44:27 - Chapter 4. How Bad is "Forbidden"? Opening Dewar Benzene
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.