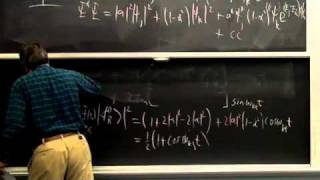24. Higher-Order Effects, Dynamics, and the NMR Time Scale
Freshman Organic Chemistry II (CHEM 125B)
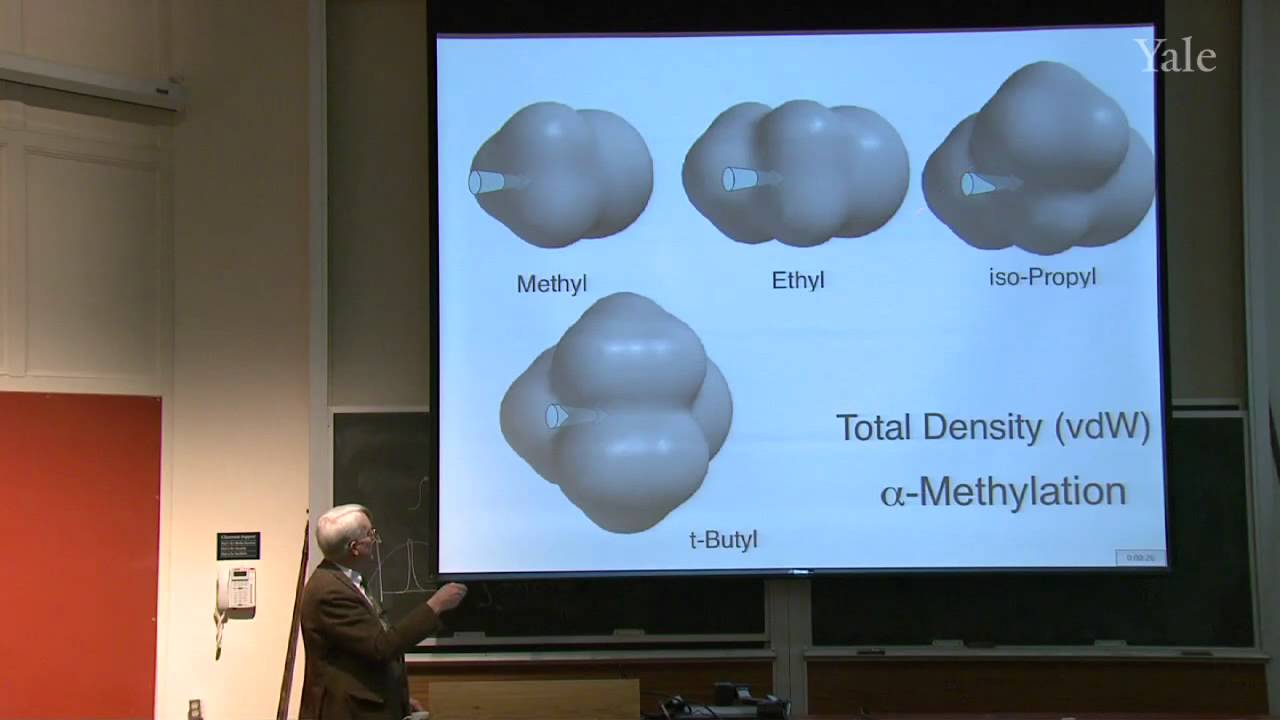
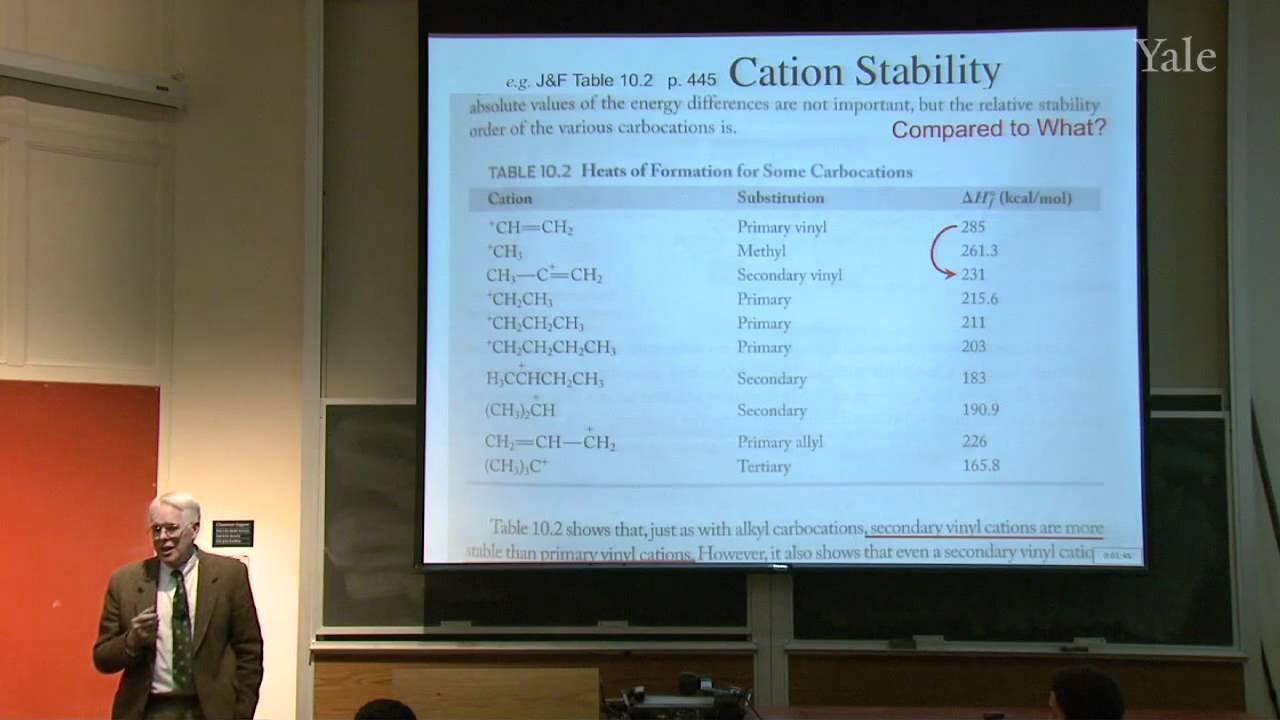
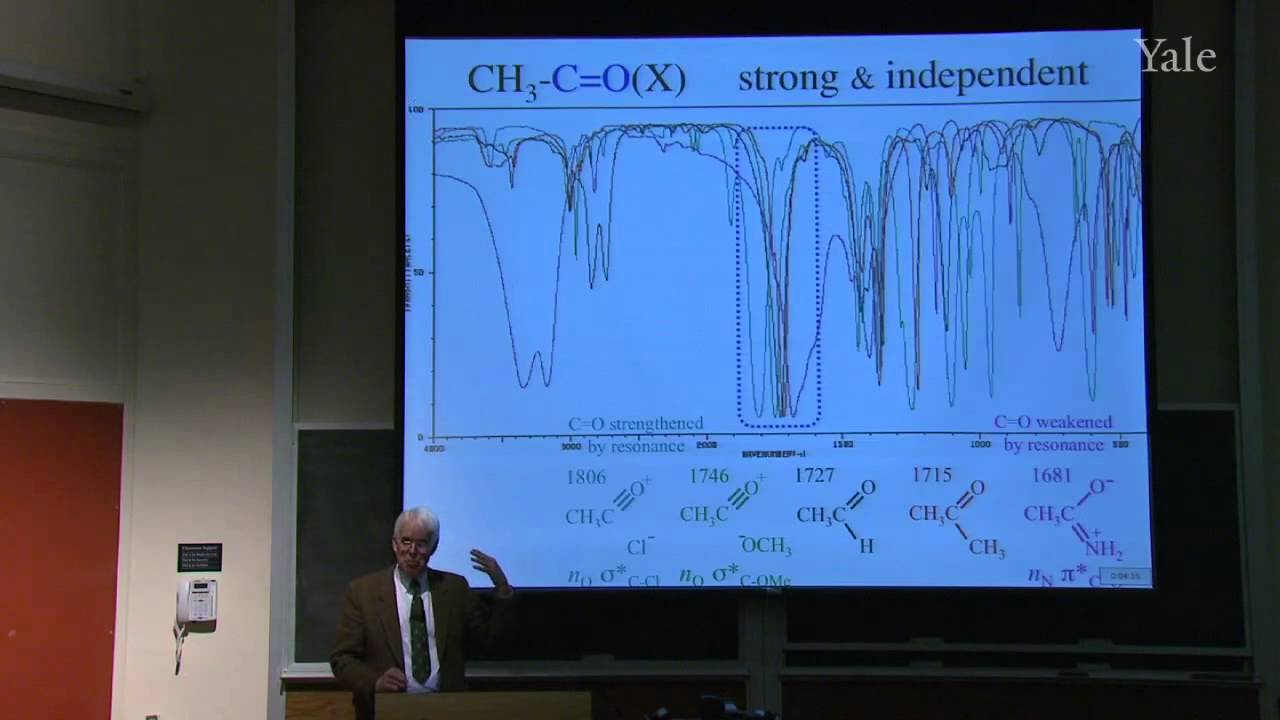
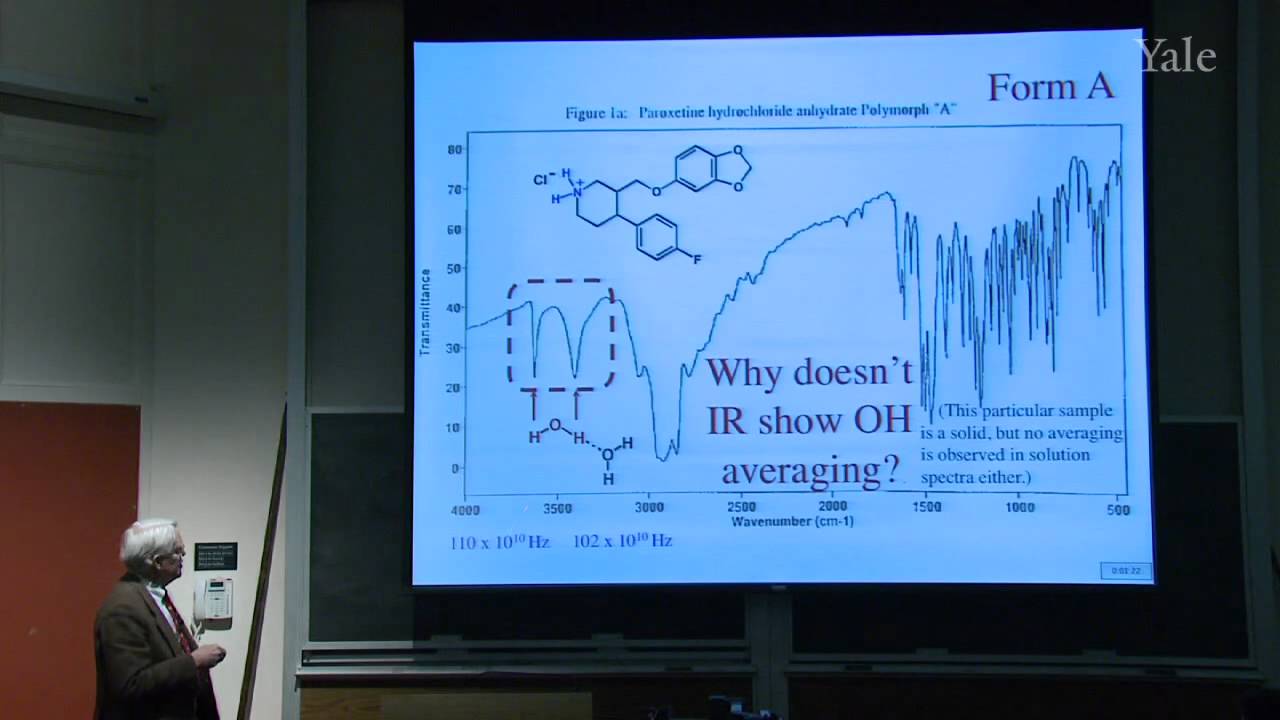
Because spin-spin splitting depends on electron spin precisely at a nucleus, splitting by a C-13 depends on its orbital's hybridization. "Higher-order effects" that give complex multiplets for nuclei with similar chemical shifts can be understood in terms of the mixing of wave functions of similar energy. Averaging of chemical shifts or spin-spin splitting may be used to measure the rate of rapid changes in molecular structure, such as changes in conformation or hydrogen bonding. Since the spectroscopic time scale depends on frequency differences, averaging is easier in NMR than in IR. A typical problem involves predicting the NMR spectrum of a compound with diastereotopic groups. In proton decoupling radio frequency irradiation of a particular proton can make it cease to split the NMR signals from nearby protons.
00:00 - Chapter 1. Hybridization and Splitting by C-13
09:39 - Chapter 2. Higher-Order Effects: Why Methane Gives a Singlet
15:57 - Chapter 3. Averaging and the NMR Time Scale
25:04 - Chapter 4. Predicting an NMR Spectrum
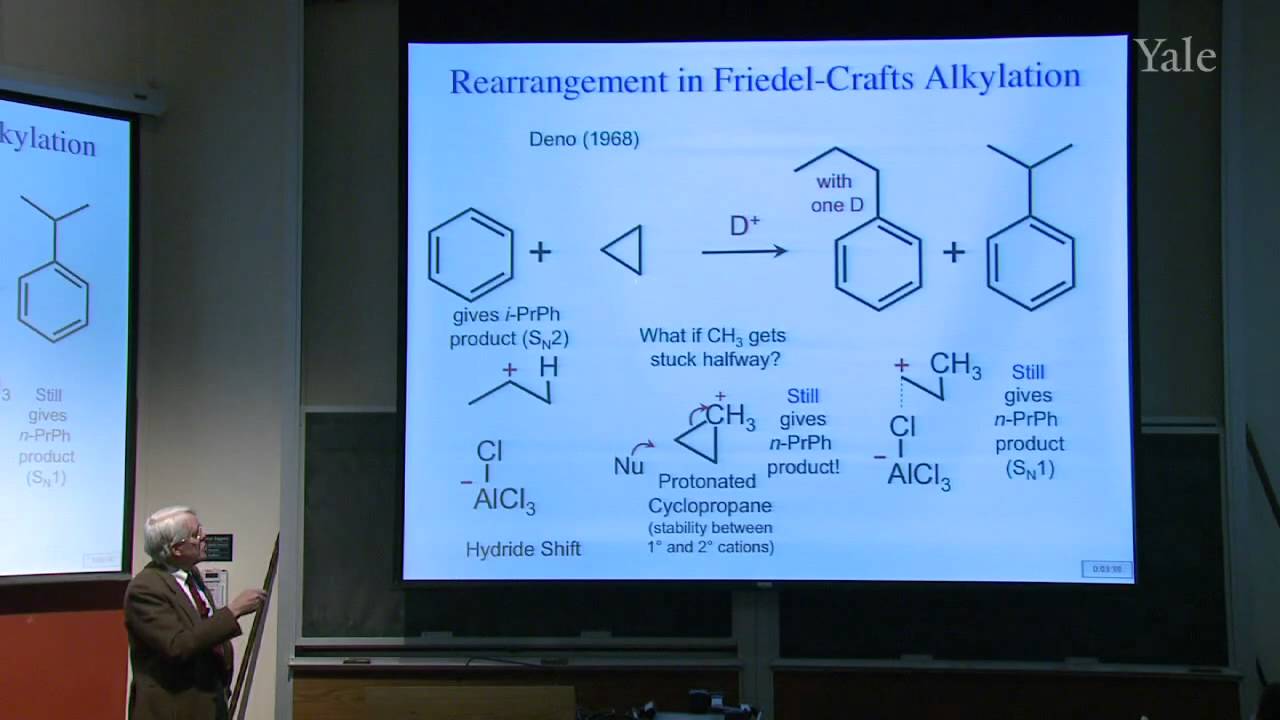
42:32 - Chapter 5. Electrophile Activation: Friedel and Crafts
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.



























![35. Acyl Insertions and [gr]α-Reactivity](https://i.ytimg.com/vi/Xb4CwzP-Hdk/maxresdefault.jpg)
![36. [gr]α-Reactivity and Condensation Reactions](https://i.ytimg.com/vi/DQfUaXLk_sw/maxresdefault.jpg)