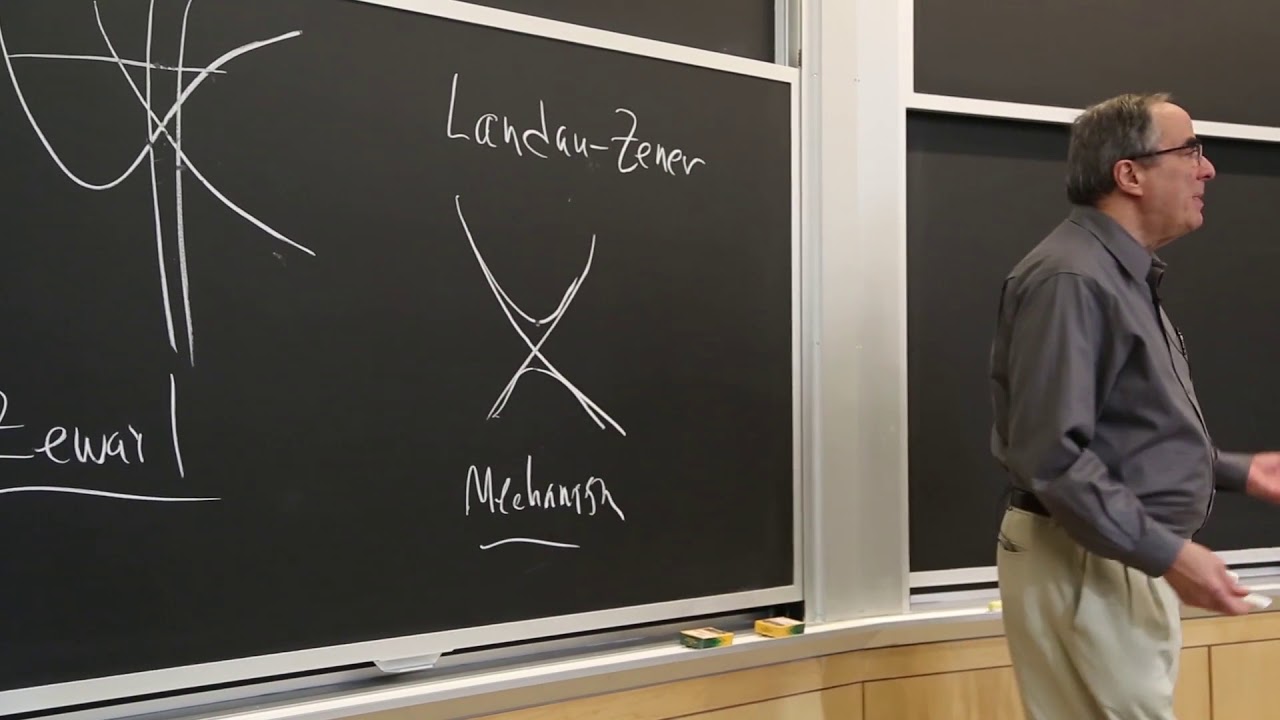37. Potential Energy Surfaces, Transition State Theory and Reaction Mechanism
Freshman Organic Chemistry (CHEM 125)
After discussing the statistical basis of the law of mass action, the lecture turns to developing a framework for understanding reaction rates. A potential energy surface that associates energy with polyatomic geometry can be realized physically for a linear, triatomic system, but it is more practical to use collective energies for starting material, transition state, and product, together with Eyring theory, to predict rates. Free-radical chain halogenation provides examples of predicting reaction equilibria and rates from bond dissociation energies. The lecture concludes with a summary of the semester's topics from the perspective of physical-organic chemistry.
00:00 - Chapter 1. The Boltzmann Factor and Entropy Against Traditional Views on Society
07:40 - Chapter 2. The Statistical Basis of the Law of Mass Action
13:13 - Chapter 3. Understanding Reaction Rates: The Potential Energy Surface and Collective Energies
29:40 - Chapter 4. Free Radical Halogenations: Predicting Reaction Equilibria and Rates
43:01 - Chapter 5. A Summary of the First Semester
Complete course materials are available at the Open Yale Courses website: http://open.yale.edu/courses
This course was recorded in Fall 2008.