17. Alkynes. Conjugation in Allylic Intermediates and Dienes
Freshman Organic Chemistry II (CHEM 125B)
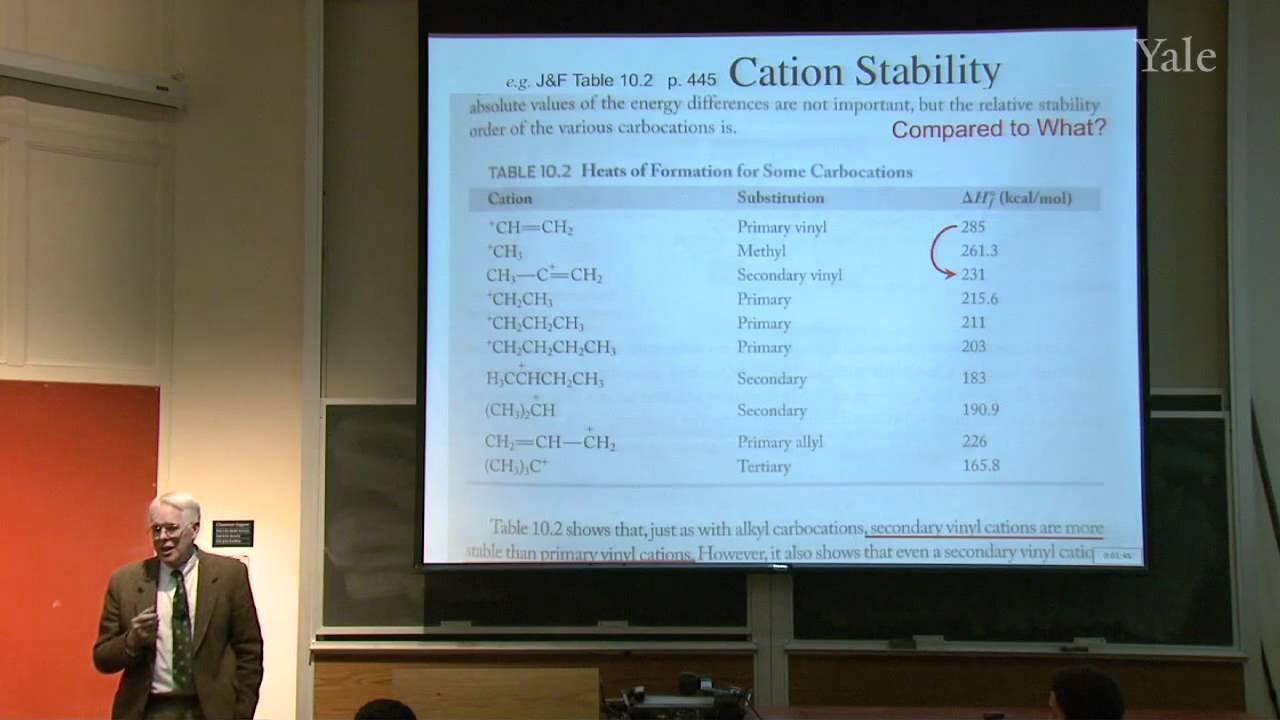
Because of their unusual acidity very strong base makes it possible to isomerize an internal acetylene to the less stable terminal isomer. Many chemical reactions may be understood in terms of localized bonds, but the special stability of conjugated systems requires considering delocalized orbitals or "resonance." Equilibrium constants, rates, and regiochemistry in systems involving allylic cations, anions, transition states, and free radicals demonstrate that allylic conjugation is worth about 13 kcal/mole. Regioselection in addition of DCl to 1,3-pentadiene reveals rapid collapse of an allylic ion pair. Allylic substitution of bromine can be favored over Br2 addition by using NBS to control Br2 concentration. Diene conjugation is worth much less than allylic conjugation.
00:00 - Chapter 1. Addition to Acetylenes: Regio- and Stereochemistry
14:05 - Chapter 2. Acidity and Isomerization of Acetylenes
20:30 - Chapter 3. When Does Conjugation Matter? Allylic Intermediates and Transition States
38:28 - Chapter 4. Allylic Radicals and Allylic Bromination
47:57 - Chapter 5. Modest Stabilization of Conjugated Dienes
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.