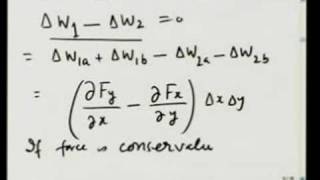7. Quantum Mechanical Kinetic Energy
Freshman Organic Chemistry (CHEM 125)
After pointing out several discrepancies between electron difference density results and Lewis bonding theory, the course proceeds to quantum mechanics in search of a fundamental understanding of chemical bonding. The wave function ψ, which beginning students find confusing, was equally confusing to the physicists who created quantum mechanics. The Schrödinger equation reckons kinetic energy through the shape of ψ. When ψ curves toward zero, kinetic energy is positive; but when it curves away, kinetic energy is negative!
00:00 - Chapter 1. Limits of the Lewis Bonding Theory
08:35 - Chapter 2. Introduction to Quantum Mechanics
16:36 - Chapter 3. Understanding Psi as a Function of Position
33:24 - Chapter 4. Understanding Negative Kinetic Energy and Finding Potential Energy
Complete course materials are available at the Open Yale Courses website: http://open.yale.edu/courses
This course was recorded in Fall 2008.