5. Solvation, H-Bonding, and Ionophores
Freshman Organic Chemistry II (CHEM 125B)
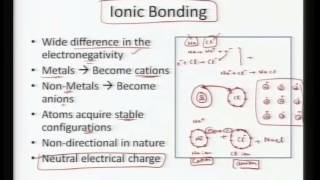
Most organic reactions occur in solution, and particularly in the case of ions, one must consider non-bonded interactions with neighboring molecules. Non-bonded interactions, including hydrogen-bonding, also determine such physical properties as boiling point. For the most part these interactions may be understood in terms of electrostatics and polarizability. Artificial or natural ion carriers (ionophores) can be tailored to bind specific ions. Energetically the ionic dissociation of water in the gas phase is prohibitively expensive.
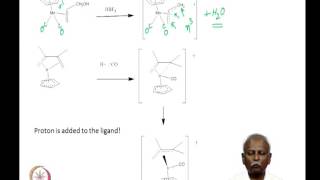
00:00 - Chapter 1. Puzzle on Alcohol Oxidation Mechanisms
02:58 - Chapter 2. Solvation, Boiling Points, and "Intramolecular Solvation"
11:45 - Chapter 3. Solvophobic Forces and Hydrogen-Bonding
28:09 - Chapter 4. Ionophores and Phase-Transfer Catalysis
45:05 - Chapter 5. Energetics of Gas-Phase Heterolysis
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.