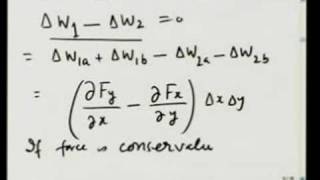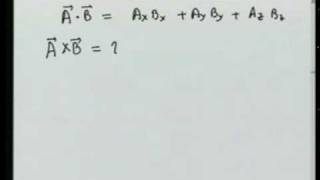33. Conformational Energy and Molecular Mechanics
Freshman Organic Chemistry (CHEM 125)
Understanding conformational relationships makes it easy to draw idealized chair structures for cyclohexane and to visualize axial-equatorial interconversion. After quantitative consideration of the conformational energies of ethane, propane, and butane, cyclohexane is used to illustrate the utility of molecular mechanics as an alternative to quantum mechanics for estimating such energies. To give useful accuracy this empirical scheme requires thousands of arbitrary parameters. Unlike quantum mechanics, it assigns strain to specific sources such as bond stretching, bending, and twisting, and van der Waals repulsion or attraction.
00:00 - Chapter 1. The 1918 Ernst Mohr Illustrations of Cyclohexane
09:40 - Chapter 2. The Invention of Conformational Analysis
22:24 - Chapter 3. Conformational Animations of Ethane, Propane, and Butane
32:05 - Chapter 4. Molecular Mechanics as an Alternative to Quantum Mechanics
40:13 - Chapter 5. Assigning Strain to Estimate Energy in Bonds
Complete course materials are available at the Open Yale Courses website: http://open.yale.edu/courses
This course was recorded in Fall 2008.