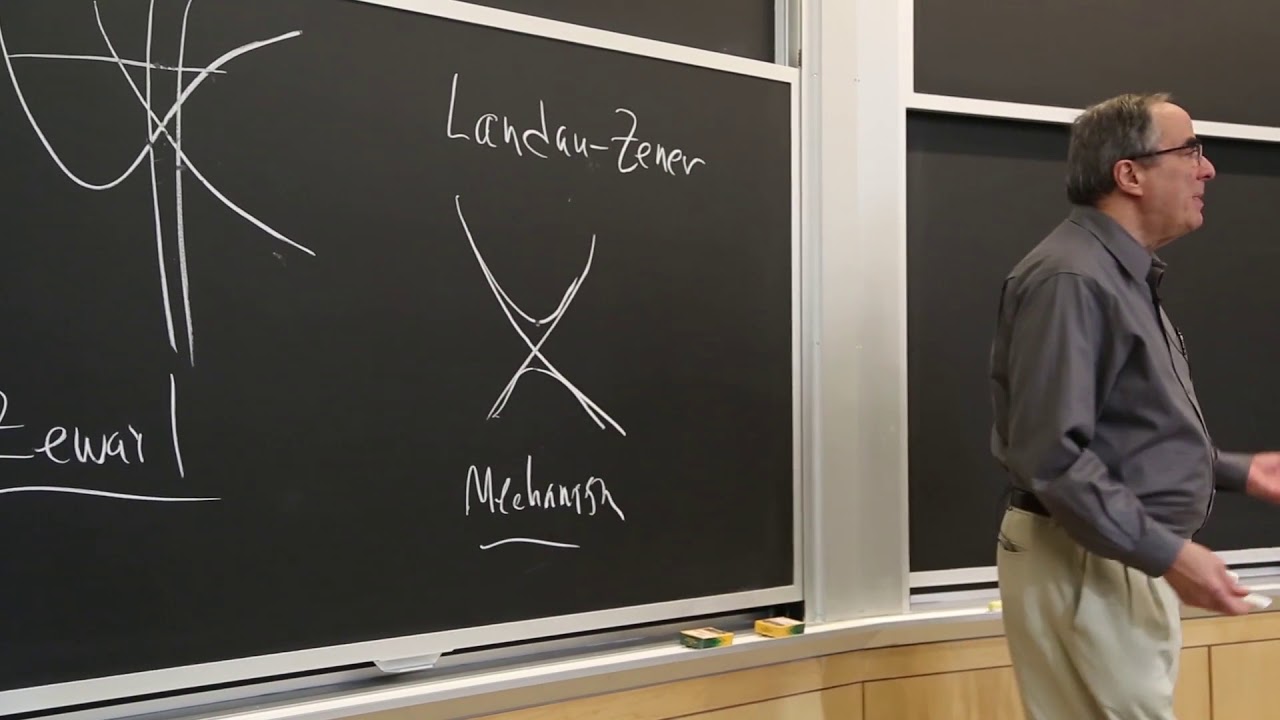32. Stereotopicity and Baeyer Strain Theory
Freshman Organic Chemistry (CHEM 125)
Why ethane has a rotational barrier is still debatable. Analyzing conformational and configurational stereotopicity relationships among constitutionally equivalent groups reveals a subtle discrimination in enzyme reactions. When Baeyer suggested strain-induced reactivity due to distorting bond angles away from those in an ideal tetrahedron, he assumed that the cyclohexane ring is flat. He was soon corrected by clever Sachse, but Sachse's weakness in rhetoric led to a quarter-century of confusion.
00:00 - Chapter 1. What is the Source of the Rotational Barrier in Ethane?
06:34 - Chapter 2. Topicity, Reactivity Difference, and Enzyme Specificity
27:49 - Chapter 3. Baeyer's Strain-Induced Reactivity Theory: Assumptions and Weaknesses
36:42 - Chapter 4. Sachse's Muddled Insights on Cyclohexane
Complete course materials are available at the Open Yale Courses website: http://open.yale.edu/courses
This course was recorded in Fall 2008.