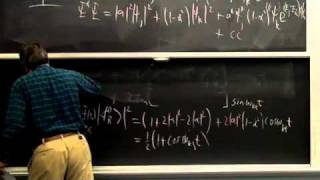20. Electronic and Vibrational Spectroscopy
Freshman Organic Chemistry II (CHEM 125B)
Time-dependent quantum mechanics shows how mixing orbitals of different energy causes electrons to vibrate. Mixing 1s with 2p causes a vibration that can absorb or generate light, while mixing 1s with 2s causes "breathing" that does not interact with light. Many natural organic chromophores involve mixing an unshared electron pair with a vacant pi orbital, whose conjugation determines color. Infrared spectra reveal atomic vibration frequencies, which are related by Hooke's law to bond strengths and "reduced" masses. Infrared spectra are complicated by the coupling of local oscillators of similar frequency to give "normal" modes. Alkane chains possess characteristic stretching and bending modes, with descriptive names, that may, or may not, absorb infrared light.
00:00 - Chapter 1. Electronic Spectroscopy: Atomic Absorption and Time Dependence
12:58 - Chapter 2. Organic Chromophores
19:38 - Chapter 3. Infrared Spectra, Hooke's Law, and Vibrational Frequency
33:09 - Chapter 4. Why IR is Complicated: Coupled Oscillators and Normal Modes
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.