16. Recognizing Functional Groups
Freshman Organic Chemistry (CHEM 125)
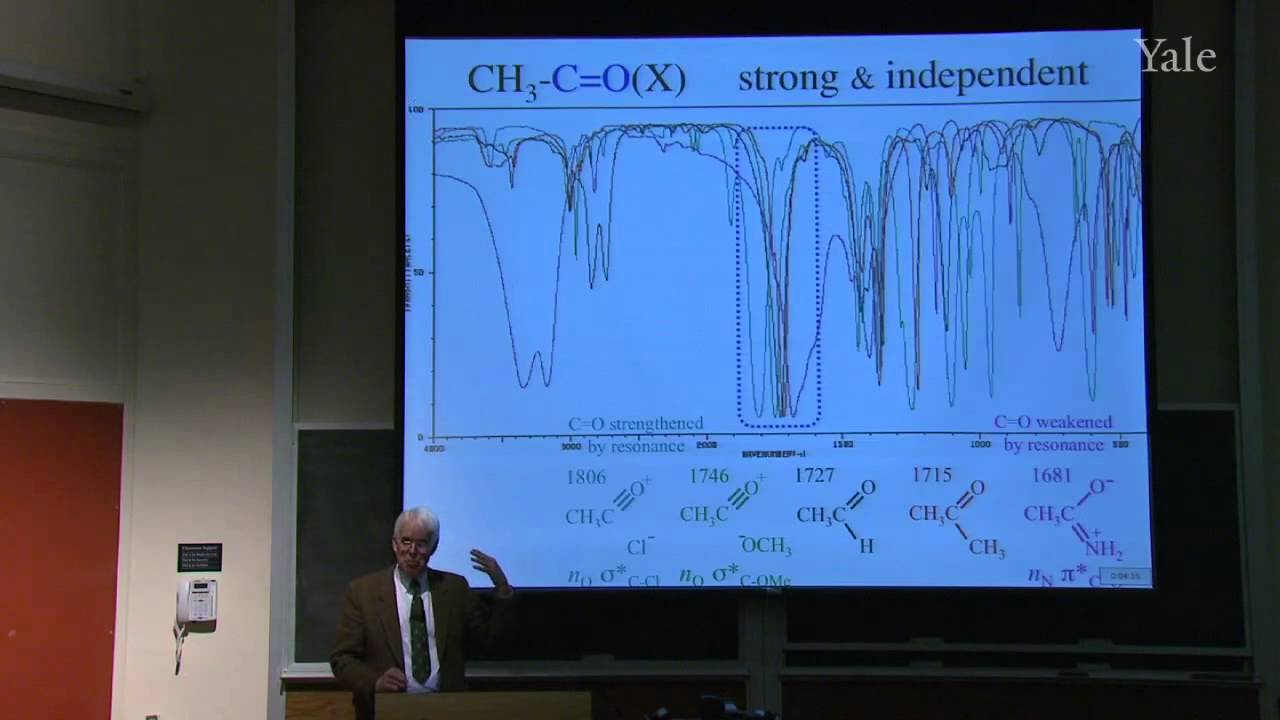
This lecture continues the discussion of the HOMO/LUMO view of chemical reactivity by focusing on ways of recognizing whether a particular HOMO should be unusually high in energy (basic), or a particular LUMO should be unusually low (acidic). The approach is illustrated with BH_3, which is both acidic and basic and thus dimerizes by forming unusual "Y" bonds. The low LUMOs that make both HF and CH_3F acidic are analyzed and compared underlining the distinction between MO nodes that derive from atomic orbitals nodes (AON) and those that are antibonding (ABN). Reaction of HF as an acid with OH- is shown to involve simultaneous bond-making and bond-breaking.
00:00 - Chapter 1. Why So High, Why So Low? The HOMO/LUMO View of Chemical Reactivity
15:19 - Chapter 2. Is BH3 an Acid or a Base?
25:38 - Chapter 3. HOMO-LUMO Mixing for Reactivity and Resonance: The Cases of HF
34:49 - Chapter 4. Comparing HF and CH3F to Distinguish Molecular Orbital Nodes
Complete course materials are available at the Open Yale Courses website: http://open.yale.edu/courses
This course was recorded in Fall 2008.