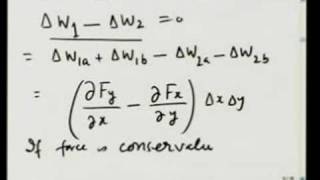13. Overlap and Energy-Match
Freshman Organic Chemistry (CHEM 125)
Professor McBride uses this lecture to show that covalent bonding depends primarily on two factors: orbital overlap and energy-match. First he discusses how overlap depends on hybridization; then how bond strength depends on the number of shared electrons. In this way quantum mechanics shows that Coulomb's law answers Newton's query about what "makes the Particles of Bodies stick together by very strong Attractions." Energy mismatch between the constituent orbitals is shown to weaken the influence of their overlap. The predictions of this theory are confirmed experimentally by measuring the bond strengths of H-H and H-F during heterolysis and homolysis.
00:00 - Chapter 1. Distance and Hybridization in the Overlap Integral
18:49 - Chapter 2. Influence of Overlap on Molecular Orbital Energy
29:45 - Chapter 3. "Inferior" Orbitals and Energy-Matching
46:59 - Chapter 4. Experimental Evidence and Conclusion
Complete course materials are available at the Open Yale Courses website: http://open.yale.edu/courses
This course was recorded in Fall 2008.