1. Mechanism: How Energies and Kinetic Order Influence Reaction Rates
Freshman Organic Chemistry II (CHEM 125B)
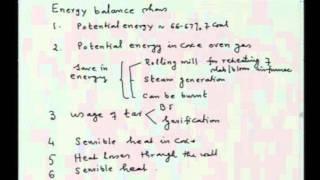
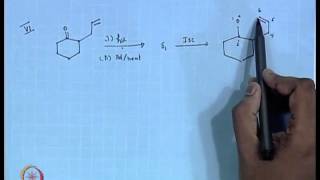
This second semester of Freshman Organic Chemistry builds on the first semester's treatment of molecular structure and energy* to discuss how reaction mechanisms have been discovered and understood. It also treats the spectroscopy and synthesis of organic molecules. Reactions and their rates can be understood in terms of reaction-coordinate diagrams involving the passage of a set of atoms through the "transition state" on the potential-energy surface. Analysis of bond-dissociation energies suggests a chain mechanism for free-radical halogenation of alkanes. Experimental determination of kinetic order provides insight into complex reaction schemes, especially when one step is rate-limiting.
00:00 - Chapter 1. Energy and the reaction coordinate
07:31 - Chapter 2. Bond Strength and the Mechanism of Free-Radical Substitution
27:23 - Chapter 3. Complex Reactions and Kinetic Order
Complete course materials are available at the Open Yale Courses website: http://oyc.yale.edu
This course was recorded in Spring 2011.